Abd-Elmagid M. Bayomy1, Yasser T. Kassem1, Mohammed M. S. Younis1, Ahmed Mahmoud Solaiman2
1Pediatric Department, Al-Azhar University, Assiut, Egypt
2Clinical Pathology Department, Al-Azhar University, Assiut, Egypt
Correspondence to: Abd-Elmagid M. Bayomy, Pediatric Department, Al-Azhar University, Assiut, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: About 60% of fetal zinc is acquired during the third trimester of pregnancy, also premature infants have a lower zinc preserver than term infants and because of immaturity; they may be less efficient at absorbing and retaining zinc for growth, so preterm neonates are at high risk of zinc deficiency. Aim of the work: This study aimed to find out the impact of cord blood zinc level on birth weight, length, and growth velocity in the first three months of life.Patients and methods: the study was conducted at Al-Azhar University hospital and Sohage teaching hospital during the period of study from June 2015 to April 2016. The sample included forty four preterm neonates and thirty six full term neonates. All the studied neonates were subjected to: detailed history taking, clinical assessment of gestational age, clinical examination, follow up period, 3 months after delivery and laboratory investigation: measurement of cord serum zinc level and hemoglobin level. Results: there was significant difference between preterm group and term group regarding both the serum zinc level and the prevalence of zinc deficiency. There was positive correlation between serum zinc level and anthropometric measures (weight, length and H.C) in preterm and term group. There was a positive correlation between serum zinc level and gestational age in preterm and term group. There was significant difference in serum zinc level among preterm group who are SGA and who are AGA. Infant with low cord zinc levels showed a statistically significance less increments in weight in comparison to infants with normal cord zinc levels and there was a statistically significance increments in length among normal zinc level group. Conclusions: normal zinc levels were found among normal birth weight and length neonates, deficient zinc levels were found among preterm and low birth weight neonates, infant with low cord zinc levels showed a statistically significance less increments in weight in comparison to infants with normal cord zinc levels and there was a statistically significance increments in length among normal zinc level group, cord zinc levels were correlated positively with birth weight, length and head circumference. There is high prevalence of low zinc level among preterm neonates. There is a highly statistically significant difference between preterm group and full term group regarding serum zinc levels being statistically higher among full terms. We recommend the importance of breast feeding on a mean of boosting zinc states in infancy. Also maternal zinc supplementation is recommended as a considerable intervention to reduce the incidence of preterm labor and other complications of pregnancy leading to it. We recommend further clinical trials to assess the role of zinc supplementation given to preterm neonates, on the growth and its effect on acquired diseases.
Keywords:
Serum zinc, Growth, Early infancy
Cite this paper: Abd-Elmagid M. Bayomy, Yasser T. Kassem, Mohammed M. S. Younis, Ahmed Mahmoud Solaiman, Cord Serum Zinc Level and Its Impact on Birth Weight, Length and Growth Velocity in the First Three Months of Life, American Journal of Medicine and Medical Sciences, Vol. 7 No. 2, 2017, pp. 47-54. doi: 10.5923/j.ajmms.20170702.02.
1. Introduction
The growth of the fetus can be regarded as a result of the interaction between its genetic potential and the intrauterine environment. Mothers who enter pregnancy in good health, with sound reproductive physiology and who have not suffered chronic illness or nutritional deprivation in childhood will have larger and healthier infants than mothers who do not have such advantages. The arrival of the newborn is highly vulnerable period during which many psychological, physiological adjustments to the extra-uterine life must be made. When a baby is born, an orderly change occurs from fetal life to extra-uterine life. It includes physical, psychological, psychosexual and cognitive changes. These changes are most prominent from one year of age even through it starts from neonatal period itself [1]. Normal growth is the progression of changes in height, weight, and head circumference that are compatible with established standards for a given population. The progression of growth is interpreted within the context of the genetic potential for a particular child [2]. Normal growth is a reflection of overall health and nutritional status. Understanding the normal patterns of growth enables the early detection of pathologic deviations (e.g. poor weight gain due to a metabolic disorder, short stature due to inflammatory bowel disease) and can prevent the unnecessary evaluation of children with acceptable normal variations in growth. Growth is an orderly process occurring in a systematic fusion. The rate and pattern of growth are specific to certain parts of the body and influenced by several factors such as: genetics, birth weight, prematurity, hormones, environments and nutritional factors. Zinc (Zn) is a transition metal belonging to group 12 of the periodic table and is considered one of the essential trace elements for plants and animals. Zinc is important for a healthy immune system, properly synthesizing DNA, promoting healthy growth during childhood, and healing wounds. The human body needs zinc to activate T lymphocytes (T cells). T cells help the body in two ways: controlling and regulating immune responses and attacking infected or cancerous cells [3]. Zinc-deficient persons experience increased susceptibility to a variety of pathogens. Zinc deficiency is common in young infants in the developing world and is associated with reduced immune-competence and increased rates of serious diseases [4]. Zinc deficiency is usually due to insufficient dietary intake. However, it may also be due to malabsorption and chronic illnesses such as diabetes, malignancy, liver disease, and sickle cell disease. Zinc deficiency was associated with loss of appetite, anemia, suppressed growth, altered cognition, diarrhea and hair loss [5].
2. Patients and Methods
This study was conducted at AL-Azhar University Hospital, from June 2015 to April 2016. This study included eighty neonates and they were divided into two groups: Group 1: Preterm neonates (their number was 44 neonates). Group 2: Full term neonates (their number was 36 neonates). Inclusion Criteria: 1- Term and preterm neonates. 2- Normal and low birth weight. 3- No indication for admission to NICU. 4- Intention to breast feed. 5-None of the mothers received large doses of zinc supplementation during pregnancy or shortly afterwards. Exclusion Criteria: 1- Congenital anomalies that may affect growth. 2- Admitted to NICU. 3- Intention for formula feed. 4-High risk pregnancy (DM, pre-eclampsia, eclampsia and severe APH). METHODS: All the studied neonates were subjected to: Detailed History taking including: Perinatal history (including: prenatal, natal, postnatal history), Socio-demographic history (including: social class, housing, education level), Maternal medical and obstetric history, Mode of delivery and outcome of pregnancy and consanguinity. Estimation of gestational age: Estimation of gestational age based on obstetric estimation, postmenstrual dates and early gestation prenatal ultrasound. All neonates underwent a gestational age examination using Ballard score in the first 24 hours of age [6]. Clinical Examination: Resuscitations: suctions, oxygen therapy and an ambo bagging when needed, Apgar score at 1st and 5th minutes and thorough clinical examination to exclude any disease or congenital anomalies may affect growth. Anthropometrics Measurement: Weight, length, head circumference (HC), and weight for length (WHO 2010). Special preterm charts will be used for evaluation of weight to gestational age among the preterm: SGA, AGA, LGA [7]. Follow up period, 3 months after delivery: With 15 days intervals between visits, all infants were followed up for the following: 1- Weight / age percentile. 2- Length / age percentile. 3- H.C / age percentile. 4- Weight / length percentile. 5- Growth velocity: change in growth over the time, one month Increments in weight and in length from birth to 3 months of life. Laboratory Investigations: Measures cord serum zinc level and hemoglobin level at birth. 1- Cord serum zinc: Cord blood sample will be collected by using sterile, dry, disposable syringes. The specimens are left to clot for one hour at room temperature in clean closed plastic tubes. Centrifugation is done after that for each specimen for 15 minutes. The clean non- hemolysed sera are separated in other clean closed plastic tubes and kept Frozen at - 20°C Until the analysis is done [8]. Manual spectrophotometry test will be used for measurement of both zinc and HB levels. A Riele Photometer 5010V5+ was used. 2- Haemoglobin (Hb): Principle: hemoglobin is the first oxidized by potassium ferricyanide into methaemoglobin which is converted into cyanmethaemoglobin by potassium cyanide. The absorbance of the cyanmethaemoglobin is monitored at 540 nm [9]. Statistical analysis: Data was analyzed using STATA intercooled version 12.1. Quantitative data was represented as mean, standard deviation, median and range. Data was analyzed using student t-test to compare means of two groups. When the data was not normally distributed Kruskal Wallis test for comparison of three or more groups and Mann-Whitney test was used to compare two groups. Qualitative data was presented as number and percentage and compared using either Chi square test or fisher exact test. Pearson correlation analysis was used to identify correlation of serum zinc level and different measurable variables. Graphs were produced by using Excel or STATA program. P value was considered significant if it was less than 0.05.
3. Results
Table 1. Characteristics of Studied Infants
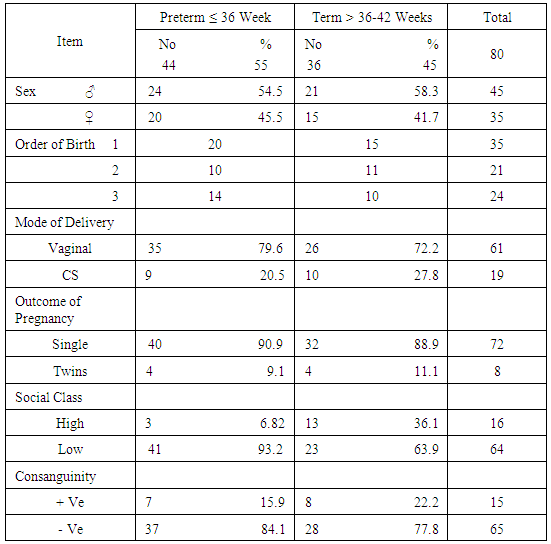 |
| |
|
Table 2. Mean ± SD of Serum Zinc Level (µg/Dl) in Relation to Birth Weight
 |
| |
|
Table 3. Mean ± SD of Serum Zinc Level (µg/dl) in Relation to Gestational Age
 |
| |
|
Table 4. Mean ± SD of Serum Zinc Level (µg/Dl) among Low Birth Weight Infants Versus Normal Birth Weight
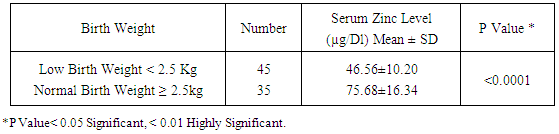 |
| |
|
Table 5. Mean ± SD of Serum Zinc Level (µg/Dl) in Relation to Birth Length
 |
| |
|
Table 6. Increment in Weight (Kg) over Time among the Study Neonates
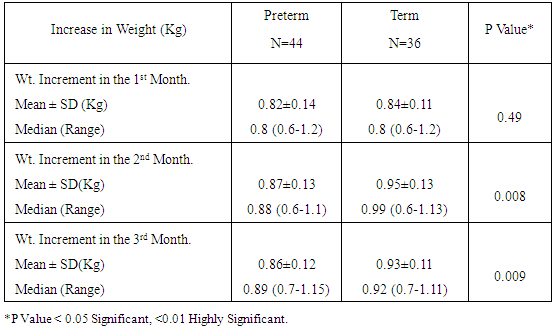 |
| |
|
Table 7. Increment in Length (cm) over Time among the Study Neonates
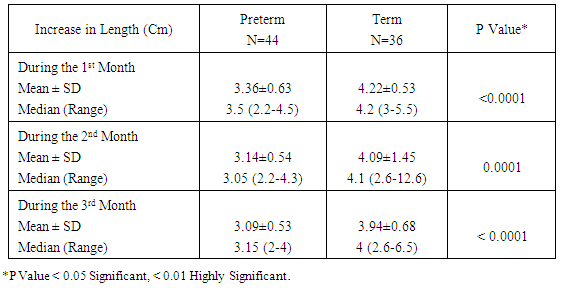 |
| |
|
Table 8. Mean ± SD of Serum Zinc Levels (µg/dl) among Term Versus Preterm Groups
 |
| |
|
Table 9. Socio-demographic Data in Relation to Mean ± SD Serum Zinc Level (µg/Dl)
 |
| |
|
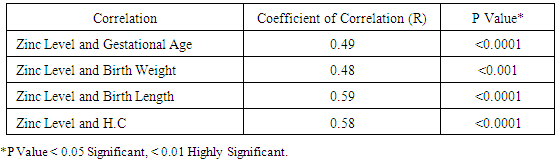
Table 10. Correlation between Cord Serum Zinc Level (µg/dl) and Gestational Age, Birth Weight, Length, and Head Circumference in Preterm Group
 |
| |
|
Table 11. Correlation between Cord Serum Zinc Level (µg/dl) and Gestational Age, Birth Weight, Length, Head Circumference in Term Group
 |
| |
|
4. Discussion
Zinc is an essential mineral that is found in almost every cell. It stimulates the activity of approximately 100 enzymes, which are substances that promote biochemical reactions in human body and is needed for DNA synthesis [10]. Zinc supports a healthy immune system, is needed for wound healing, and has an important role in normal growth and development during pregnancy, infancy, childhood, and adolescence [11]. It plays an important role during pregnancy; low dietary zinc intake or low maternal plasma zinc are associated with an increased risk of LBW and preterm delivery; it has been also reported to correlate with pregnancy complications such as prolonged labor, hypertension, postpartum hemorrhage, spontaneous abortion, and congenital malformation [12]. Regarding our choice of the studied infants, all enrolled infants were adequately breast fed and healthy. None of the mothers received zinc during pregnancy or after delivery to exclude the possible effect of zinc on growth of their babies. Term and preterm babies were enrolled in the study to explore the relation between gestational age and zinc level. Infants with congenital anomalies that may affect the growth were excluded from our study. Infants with diseases and required admission to NICU or received drugs may affect growth or zinc levels also excluded from this study. As regard the enrollment of infants during the 3 months study period, 44 infants were preterm (55%) 24 of them were males (54.5%) and 20 of them were females (45.5%), their gestational age ranged between 32-36 weeks and their birth weight ranged from 1.300 -2.900 Kg. On the other hand 36 infants were full term (45%) 21 of them were males (58.3%) and 15 of them were females (41.7%), their gestational age ranged between 37-40 weeks and their weight ranged from 2.240 -4.100 Kg. All infants not admitted to NICU and exclusively breast fed to exclude the effect of possibly fortified formula milk on growth of infants. Forty four infants were LBW infants (wt < 2.500 Kg), 39 of them were preterm and 5 were full term. Thirty five of the preterm infants delivered by NVD and only 9 delivered by CS, on the other hand 26 of the full term infant delivered by NVD and 10 delivered by CS. The outcome of pregnancy in the studied infant was single except in 8 cases were twins (4 preterm and 4 full term). Sixty four of the studied infants had a low social class (41 preterm, 23 full terms) and 16 had a high social class (3 preterm, 13 full terms). No consanguinity in 65 cases (37 preterm, 28 full terms) and positive consanguinity occurs in 15 cases (7 preterm, 8 full terms). More than half of the parents of studied infants were illiterate (45 cases), 24 cases with middle education level and 11 cases with high education level. About the relation between cord serum zinc level and birth weight , we found a direct relationship between birth weight and zinc level, where LBW group had the lowest zinc levels (42.09μg /dl), while infants of birth weight > 4Kg had the highest zinc levels (97.00μg /dl), and this relation is statistically highly significant (p value: <0.0001). This result are in agreement with results obtained by Jeswani and Vani, 1991, [13] who found a direct relationship between weight (keeping gestational age constant) and serum zinc level in cases of neonates (Jeswani and Vani, 1991), [13]. LBW infants had the lowest levels of cord serum zinc level because LBW infants had lower body stores of zinc. Infants born prematurely are especially vulnerable to limited body stores [14]. Regarding the relation between cord serum zinc level and gestational age our results showed that the preterm infants had the lowest zinc levels (47.07μg/dl) while full term infants had a higher zinc levels (94.6μg/dl) and this is close to that reported by Jeswani who found that there was a direct relationship between the gestational age and serum zinc level in the cord blood of neonates [13]. Zinc deficiency among preterm infants may be due to maternal zinc deficiency, inadequate trans placental transfer of zinc, inadequate storage and release of zinc from neonate’s liver and increased tissue utilization of zinc. A low intake of dietary zinc early in pregnancy is associated with approximately two fold increase in the risk of low birth weight (LBW), and a greater than threefold increase in the risk of preterm delivery [15]. Regarding relation between cord serum zinc levels and birth weight, length and H.C, we found a highly statistical significant direct relationship between zinc levels and birth weight (p <0.0001), zinc levels and birth length (p <0.0001) and zinc levels and H.C (p <0.0001), these results were in agreement with Diaz- Gomez et al, 2005, El- Tobgi et al, 2003, and Zaki et al, 2005 [16-18], who found that cord serum zinc levels correlated significantly with birth weight, length and head circumference. In our study we found a statistically significant increase in body weight of term neonates compared to preterm during 2nd and 3rd month follow up period p value (0.008) and (0.009). There was a non-statistical significant increase in the body weight during the 1st month of life among term group and this can be explained by minimal increase in body weight during 1st 15 days of life due to 10% physiological loss of birth weight during this period and then increase in body weight during 2nd and 3rd month of life and these results was in agreement with results obtained by Diaz- Gomez et al, 2003, [16]. In our study we found that a statistical significant increment of length of term group within the 1st 3 months of life more than that for preterm group within the same period, p value (< 0.001) and these results were in agreement with results of El- Tobgi et al, 2005, [17]. Regarding the increments in weight in the first 3 months of age in relation to zinc levels, we found a statistically significant increase in the weight for normal zinc level group during the 1st 3 months follow up period than that in low zinc level group within the same period (p value: 0.0001) but the increment in weight during the 1st month was more in normal zinc level group than the low zinc level group and the increment during the 2nd and 3rd month is more in low zinc level group than normal zinc level group and this can be explained by the presence of LBW infants between the low zinc level group (whose increment in weight is more than preterm infants) and all our studied infants breast fed without any supplementation or fortified formula that may lead to decrease growth in normal zinc level group. However, infants with normal zinc level were significantly increased in weight during the whole 3 months follow up period more than that in zinc deficient group. Low zinc level group mostly preterm and having low birth weight. Preterm birth is often associated with nutritional compromise and impaired growth performance [16]. Preterm infants have a high risk of zinc, copper, and other micronutrient deficiencies and are frequently growth-retarded. There are multiple contributing factors that explain this. As a consequence of shorter gestation and the immaturity of the gastrointestinal tract, these infants have lower body stores. There was statistically significant positive correlation between the serum zinc level and head circumference in preterm and term group at birth. Premature infants have a high nutrient demand and low body Zinc stores because of rapid postnatal growth and an increased risk of inter-current diseases, which means that the intake of nutrients may be inadequate during the first months of life. Regarding the increments in length during the first 3 months of age in relation to zinc levels we found a statistically significant increase in length among normal zinc level group in comparison to low zinc level group within the first 3 months of life (p value < 0.0001) but the increment in length during the 3 month follow up period in low zinc level group is more than normal zinc level group and this can be explained by the presence of LBW infants between the low zinc level group (whose increment in length is more than preterm infants) and all our studied infants breast fed without any supplementation or fortified formula that may lead to decrease growth in normal zinc level group. Regarding to the mean ± SD of increment in head circumference (cm) among the study neonates from birth through 3 months of age, the mean ± SD of increment in H.C (cm) in term group was more than preterm group and it is statistically highly significant (p value: <0.0001), We found that a highly statistically significant difference between preterm group and term group as regard cord serum zinc level (µg/dl), (p value <0.0001). Also the prevalence of zinc deficiency among term group was 11.1% compared to 72.27% among preterm group (p value <0.0001) and these results in consistent with results obtained by Jeswani RM and Vani SN, (1991), [13], who found statistically positive correlation between gestational age, body weight, length, head circumference and cord serum zinc level (µg/dl). Regarding to Hb level in preterm and full term group, there was a highly statistically significant difference between the preterm and full term group regarding Hb level. The mean ± SD of Hb in preterm group was 10.14 ±1.12 and the mean ± SD of Hb in full term group was 15.06 ± 1.47 (p value <0.0001). Regarding serum zinc level in relation to Hb level, the lowest Hb <11 gm/ dl associated with low zinc level 44.00 ±7.45 and high Hb level > 15 gm/ dl associated with normal zinc level 86.50 ±11.80. The relation is highly statistically significant (p value: < 0.0001). This alteration in zinc level in relation to Hb level could be explained by alterations in dietary levels of one mineral can alter transfer across the gut or the placenta of another mineral, which may have an important bearing on physiological effects of deficiencies. In the present study we found that a statistically significant difference (p value = 0.0004) between the high social class and low social class neonates and between the educational level of mothers (high, middle and low) in relation to cord serum zinc levels (µg/dl). This explained by inadequate dietary intake of absorbable zinc by low social class population and mothers with middle and low educational levels and also may be due to impaired absorption of zinc as a result of interaction with other dietary components such as phosphorus, calcium and phytates. In our study we found that a highly statistical significant positive correlation between zinc level (µg/dl) and gestational age, birth weight, length and head circumference in term and preterm neonates (r = 0.49, 0.48, 0.59, 0.58 in preterm) and (r = 0.68, 0.58, 0.54, 0.54 in term infants). These results were in agreement with results of Diaz- Gomez et al, 2003, El- Tobgi et al, 2005, and Zaki et al, 2003, [16-18], who found a statistical significant positive correlation between gestational age, birth weight, length and serum zinc level (µg/dl).
References
| [1] | Hillman N, Kallapur SG and Jobe A, (2012). Phsiology of Transition from intrauterine to extrauterine life, clin perinatol. 2012, 39(4):769-783. |
| [2] | Lifshitz F and Cervantes CD, (1996). Short stature. In: Pediatric Endocrinology, Lifshitz, F (Ed), Marcel Dekker, New York 1996. P, 3. |
| [3] | Kaltenberg J, Plum LM, Ober- Blobaum JL, Honscheid A, Rink L, Hasse H, (2010). Zinc signals promote IL-2-dependent proliferation of T cells. Eur J Immunol. May; 40(5): 1496-503. |
| [4] | Sur D, Gupta DN, Mondal SK, Ghosh S, Manna B, Rajendran K, (2003). Impact of zinc supplementation on diarrheal morbidity and growth pattern of low birth weight infants in Kolkata, India: A randomized, double-blind, placebo-controlled, community-based study. Pediatrics; 112:1327-1332. |
| [5] | Hambidge KM (2000). Human zinc deficiency. Journal of Nutrition, 130: 1344s-1349s. |
| [6] | Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R, (1991): New Ballard score, expanded to include extremely premature infant. J Pediatr., 119:417-423. |
| [7] | Fenton TR, (2003). A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. Dec 16; 3:13. |
| [8] | Burtis A and Ashwood R, (1999). Tietz Textbook of clinical chemistry, 3rd ed AACC 1999 |
| [9] | van Kampen EJ and Zijlstra WG, (1961). Clin. Chim. Acta; 6:538-544. |
| [10] | Food and Nutrition Board, Institute of Medicine (2001). Dietary Reference Intakes For Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, And Zinc. National Academy Press, Washington, D.C. |
| [11] | Prasad AS, (1995). Zinc: An overview. Nutrition; 11:93-99. |
| [12] | Gibson RS, (1994b). Zinc nutrition in developing countries. Nutr.Res.Rev. 7:151-173. |
| [13] | Jeswani RM and Vani SN, (1991). A study of serum zinc levels in cord blood of neonates and their mothers. Indian J Pediatr. Sep-Oct; 58(5):683-6. |
| [14] | Higashi A, Matsuda I, Masumoto T, Saikusa H, Yabuso M, Oka Y, (1985). Serum zinc and copper concentrations in low birth weight infants during first three months of life; correlation to birth weight and different feedings. Tohoku J Exp Med. Jul; 146(3): 253-63. |
| [15] | Scholl TO, Hediger ML, Schall JI, Fischer RL, Khoo CS (1993). Low zinc intake during pregnancy: its association with preterm and very preterm delivery. Am J Epidemiol. 15; 137(10):1115-24. |
| [16] | Díaz-Gómez NM1, Doménech E, Barroso F, Castells S, Cortabarria C, Jiménez A, (2003). The effect of zinc supplementation on linear growth, body composition, and growth factors in preterm infants, 111(5 Pt 1):1002-9. |
| [17] | El-Tobgi MM, Gabr AA, Boseila SA, Shafei HF, Fouda AR, Khalifa AG, El-Sheikh OM, Salem SI, (2005). Effect of supplementation with zinc and iron on growth, development and morbidity in infants in an urban neighbourhood in Cairo, Egypt. Med. J. Cairo Univ., Vol 73, No. 4, December: 737-745, 2005. |
| [18] | Zaki ST, El- Shebini SM, El-Shobaki FA, (2003). Role of iron and zinc supplementation in the growth retardation and iron deficiency anemia in Egyptian children. Med. J. Cairo Univ., Vol. 71, No. 1 (suppl.) March: 183-187. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML









