-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(2): 37-46
doi:10.5923/j.ajmms.20170702.01

Comparative Study between Continuous Positive Airway Pressure with or without Surfactant in Management of Preterm Babies with Respiratory Distress Syndrome
Hosny M. A. El-Masry1, Alaa-Eldin A. Hassan1, Amira M. M. Hamed1, Emad M. Hammad2
1Pediatric Department, Al-Azhar University, Assiut, Egypt
2Pediatric Department, Assiut University, Assiut, Egypt
Correspondence to: Hosny M. A. El-Masry, Pediatric Department, Al-Azhar University, Assiut, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Mechanical ventilation is often initiated after endotracheal intubation for surfactant administration, without consideration of the fact that many infants who are able to breathe spontaneously could be supported with NCPAP only. Aim of work: The aim of study was comparing effect of use of nasal continuous positive airway pressure (NCPAP) with and without surfactant therapy in management of preterm neonates with RDS on subsequent outcome. Patients and methods: Our study is randomized controlled trial including all neonates with gestational age 28 – 32 weeks born at Women Health University Hospital and Al-Azhar Assiut University Hospital and admitted to the Neonatal Intensive Care Unit of Assiut University children Hospital and Al-Azhar Assiut University Neonatal ICU during the period from January 2015 to the end of December 2015), and the postnatal age between 15 minutes and 48 hour, suffering from respiratory distress with evidence of increased work of breathing (tachypnea, intercostal retractions, nasal flaring, or grunting) and need supplemental oxygen. These preterm neonates were continuously evaluated for the presence of RDS by clinical and radiographic criteria and requiring supplemental oxygen by nasal continuous positive airway pressure (NCPAP). Eligible infants were initially placed on NCPAP of 5 cm H2O, and a flow of 8-10 L/min of oxygen and immediately a randomization selection of cases either for early NCPAP plus surfactant (treatment group) or to early NCPAP alone (control group). Randomization done according to their admission in which first case received NCPAP, 2nd received surfactant plus NCPAP. Results: This study demonstrates that preterm neonates born at 28 to 32 weeks’ gestation supported with NCPAP only compared with addition of surfactant therapy in patient with moderate to severe respiratory distress using INSURE method(intubation- surfactant administration followed by extubation to NCPAP), there were no significant differences seen in the two groups in terms of late morbidity or mortality and the effect appear only in improvement of clinical sign in the form of improvement of tachypnea, oxygenation, acid base disturbance, slight reduction in need for mechanical ventilation, duration of stay on mechanical ventilator(MV), reduction of air leak syndrome, occurrence of 3rd degree intra-ventricular hemorrhage or persistence of PDA. At the same time there were no significant difference in imaging study including improvement in chest X ray, also duration of oxygen therapy and so development of broncho-pulmonary dysplasia, duration of hospital stay, and mortality, thus reduced costs associated with the administration of surfactant.
Keywords: CPAP, Surfactant, Preterm, RDS
Cite this paper: Hosny M. A. El-Masry, Alaa-Eldin A. Hassan, Amira M. M. Hamed, Emad M. Hammad, Comparative Study between Continuous Positive Airway Pressure with or without Surfactant in Management of Preterm Babies with Respiratory Distress Syndrome, American Journal of Medicine and Medical Sciences, Vol. 7 No. 2, 2017, pp. 37-46. doi: 10.5923/j.ajmms.20170702.01.
1. Introduction
- Respiratory Distress Syndrome (RDS) resulting from a deficiency of pulmonary surfactant is the most frequent clinical respiratory disorder in preterm infants. Over the last decade, because of improvements in neonatal care and increased use of antenatal steroids and surfactant replacement therapy, mortality from RDS has dropped [1]. The incidence of RDS is inversely related to gestational age and birth weight. It occurs in 60-80% of infants < 28 wk of gestational age, in 15-30% of those between 32 and 36 wk, and rarely in those >37 wk. [2]. RDS occurs in ~50% of infants with birth weight between 501 and 1500 g RDS approximately doubled for each week of gestation below 37 weeks. Among respiratory support techniques, nasal continuous positive airway pressure (NCPAP) and mechanical ventilation (MV) are known for their effectiveness in reducing the mortality and morbidity rates associated with RDS. Moreover, early application of NCPAP and early treatment with surfactant are effective in decreasing the need for MV, with its related adverse effects. Unfortunately, these results are not always taken into account in neonatal intensive care units, and MV is often initiated after endotracheal intubation for surfactant administration, without consideration of the fact that many infants who are able to breathe spontaneously could be supported with NCPAP only [3]. The use of NCPAP is based on the principle of alveolar distension, with maintenance of its patency during expiration having in this way a similar effect to surfactant. Here there is debate about the need to administer prophylactic surfactant to newborns undergoing NCPAP and particularly those at a lesser risk of developing RDS3. There are only a few studies in the literature comparing the advantages of using the INSURE method with the isolated use of NCPAP [4].Aim of the work: The aim of study was comparing effect of use of nasal continuous positive airway pressure (NCPAP) with and without surfactant therapy in management of preterm neonates with RDS on subsequent outcome of morbidity, mortality, need of mechanical ventilation, hospital stay and so on.
2. Patients and Methods
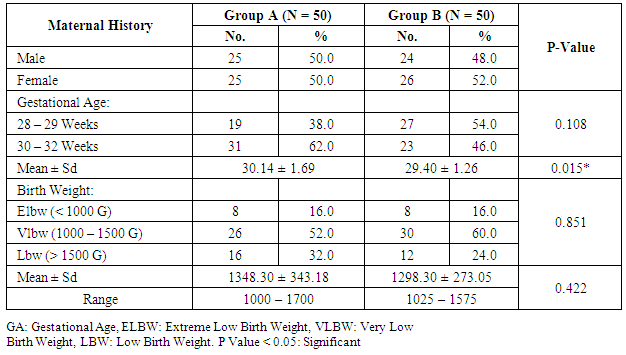
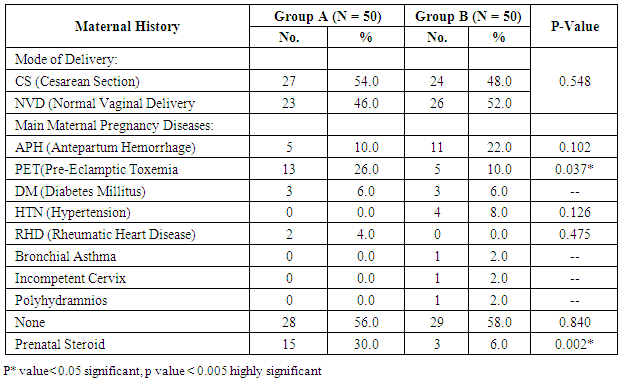
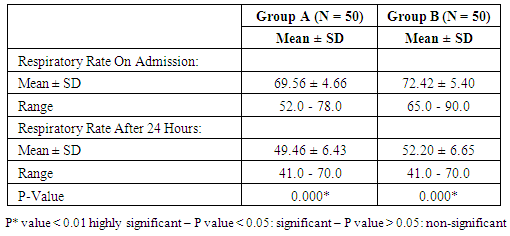
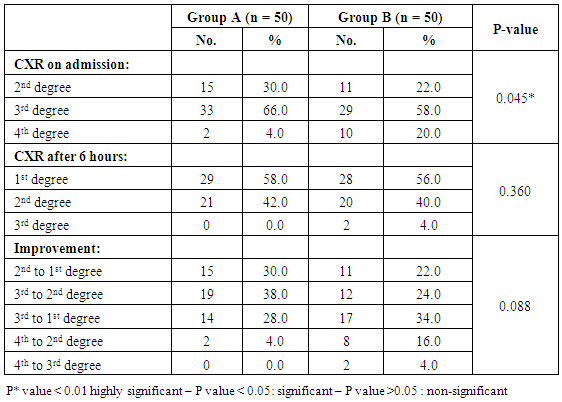
- Our study is randomized controlled trial including all neonates with gestational age 28 – 32 weeks born at Women Health University Hospital and Al-Azhar Assiut University Hospital and admitted to the Neonatal Intensive Care Unit of Assiut University children Hospital during the period of study from start of January 2015 to the end of December 2015.(A) Inclusion criteriaAll neonates with gestational age 28 - 32 weeks assessed by New Ballared score and ultrasosic estimation [5], postnatal age between 15 minutes and 48 hour, suffering from respiratory distress with evidence of increased work of breathing (tachypnea, intercostal retractions, nasal flaring, or grunting) and need supplemental oxygen.(B) Exclusion criteria1. Neonats delivered to mothers with premature ruptured membranes more than 18 hours.2. Gestational age less than 28 weeks & more than 32 weeks.3. Congenital anomalies of the heart leading to respiratory distress4. Congenital anomalies affecting respiratory system e.g. diaphragmatic hernia, hypoplastic lung, congenital lobar emphysema…. etc5. Meconium aspiration syndrome.At birth, all newborn infants admitted to the NICU with GA of 28 to 32 weeks were resuscitated in a standardized manner beginning with 100% oxygen according to Kattwinkel et al., (2011), [6]. The fraction of inspired oxygen (FIO2) was adjusted thereafter to maintain the pulse oximetry saturation (SpO2) between 90% and 95%. Between 15 minutes and 48 hour of life, infants were continuously evaluated for the presence RDS by clinical and radiographic criteria and requiring supplemental oxygen by NCPAP. RDS was defined as clinical respiratory distress with increased work of breathing in the presence of chest radiographic (CXR) evidence of lung field granularity, small lung volumes and air bronchograms. Eligible infants were initially placed on NCPAP of 5 cm H2O, and a flow of 8- 10 L/min of oxygen and immediately a randomization selection of cases either for early NCPAP plus surfactant (treatment group B) or to early NCPAP alone (control group A). Randomization was done according to neonatal admission in which first case received NCPAP, 2nd received surfactant plus NCPAP. Infant assigned to the treatment group were temporarily intubated for surfactant administration. Before surfactant administration, correct position of the endotracheal tube was determined by length of the tube at the lip, symmetry of breath sounds, and chest wall rise. A modified natural bovine lung surfactant was administered at a dose of 100 mg/kg in 2 aliquots, 2 minutes apart. PPV was administered by using ambu bag for 1 minute after each aliquot with the previously described pressures, followed by extubation to NCPAP with a pressure of 5 cm H2O and a humidification temperature of 36.7-37.3°C. All participating infants received a loading dose of caffeine (20 mg/kg intravenously), followed by a maintenance dose of 5 mg/kg every 24 hours, as long as they remained on NCPAP. Parental consent was obtained before enrollment. Arterial blood gas (ABG) analysis was performed. CPAP was considered to be successful if the respiratory distress improved and the baby could be successfully weaned off CPAP. The criteria for weaning was absence of respiratory distress (minimal or no retractions and respiratory rate between 30 and 60 per minute) and SpO2> 90% on FiO2< 30% and PEEP < 5 cm of water. Mechanical ventilation was considered for failure of CPAP.The criteria for MV included:1) a fraction of inspired oxygen (FIO2) greater than 0.60 required to maintain an indicated saturation of peripheral oxygen (SpO2) at or above 90% for 1 hour2) PaO2< 50mmHg3) PaCO2> 60 mmHg4) pH < 7.25 with FiO2 > 0.6, documented by a single measurement of blood gases within 1 hour before intubation5) Hemodynamic instability, defined as:a. a blood pressure that was low for gestational ageb. Poor perfusion, or bothc. Requiring volume or pressure support for a period of 4 hours or more.6) Clinical deterioration:a. Increased respiratory distress including severe retractions on PEEP >7 cm of water; b. Prolonged (>20 seconds) or recurrent apneas (>2 episodes within 24 hours associated with bradycardia) requiring bag and mask ventilation. Infants who were intubated within the first 48 hours after birth were to receive surfactant.All infants who met treatment-failure criteria were eligible to receive rescue surfactant therapy. Rescue doses of surfactant (doses after the initial dose in the treatment group) were administered under specified guidelines as follows. Infants in the control group who met treatment failure criteria were intubated and placed on MV and subsequently received an initial dose of surfactant in a standardized manner as described for the treatment group. Weaning criteria: Gradual weaning of the ventilator settings then took place while maintaining SpO2 within the pre-established ranges. If FIO2 was weaned to 0.30, no additional doses of surfactant were administered; if FIO2 was 0.30, additional doses were given every 6 hours until a total of 4 doses were administered. Surfactant was not administered beyond 72 hours of age. Infants in the treatment group who met treatment-failure criteria were intubated and placed on MV, and surfactant was administered in a standardized manner as previously described with a maximum of 4 doses, including the dose administered at the time of randomization. It was not necessary to wait 6 hours from study treatment (either surfactant or control) to administer the first subsequent dose of surfactant if other clinical criteria were met. Infant variables evaluated included birth weight, sex, gestational age, clinical sign using Down's score, chest X-ray, and blood gas, associated complication and mortality.Statistical analysis: Data were given as mean and range or number (percentage) and were analyzed with SPSS software (SPSS for Mac, version 16.0, Chicago, Illinois, USA). The baseline characteristics of the two groups were compared using Student’s t-test for parametric and the chi-squared test for nonparametric comparisons. P values <0.05 were considered statistically significant.
3. Results
|
|
|
|
|
|
4. Discussion
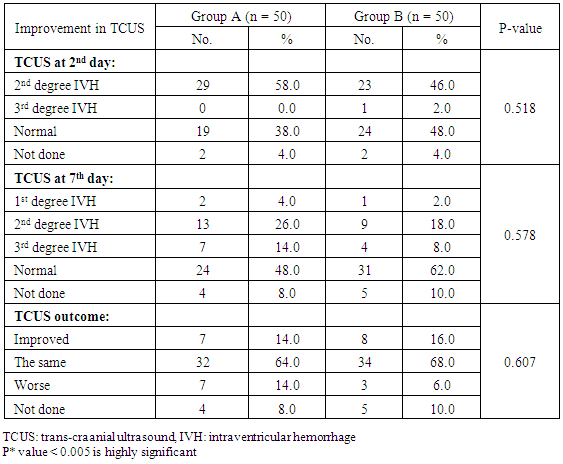
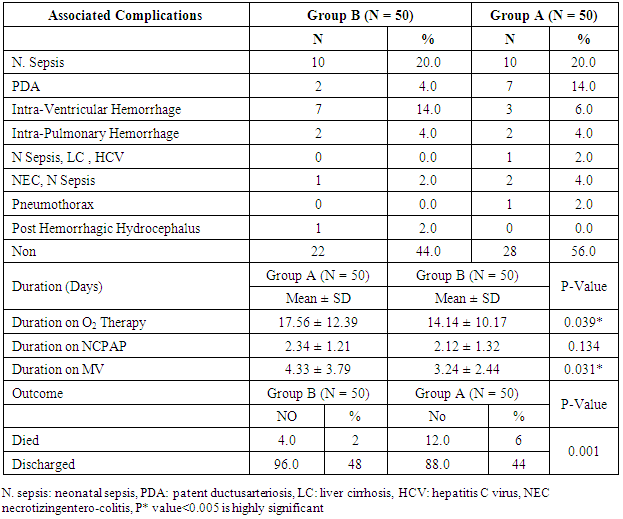
- Respiratory Distress Syndrome is the most common respiratory disorder in preterm infants [1]. It is the most important cause of morbidity and mortality in preterm infants [7] and [8]. Similarly in a study from Egypt, Respiratory Distress Syndrome was reported to be the main cause of neonatal deaths. Various approaches have been tried to improve the outcome of those infants, including: (1) the use of antenatal steroids to enhance pulmonary maturity, (2) appropriate resuscitation and immediate use of continuous positive airway pressure for alveolar recruitment; and (3) early administration of surfactant to minimize damage to the immature lungs. Use of mechanical ventilation may be associated with some complications which are mostly iatrogenic, such as barotraumas and BPD. Ventilation also involves insertion of an endotracheal tube which is associated with long term complications such as subglottic stenosis and respiratory infections. There has been increased tendency to the use of CPAP as a primary therapy, because it is a gentler mode of respiratory support in RDS. This will improve mortality rate and reduce the occurrence of long-term respiratory complications [9]. In the present study we evaluated the role of Nasal Continuous Positive Airway Pressure (NCPAP) therapy with and without surfactant in management of respiratory distress syndrome in preterm infants. In this study we found that there was increased number of neonates of mother with pre-eclamptic toxemia in group (A) who received NCPAP only than those received NCPAP with surfactant group (B). The frequency in NCPAP only (group A) to NCPAP with surfactant (group B) was 26% vs. 10% with p value < 0.05. This could be explained by that Pre-eclamptic toxemia is a stress factor that helps in increasing lung maturity and so decease incidence of RDS. This is in agreement with Carlo and Ambalavanan, (2011), [2] who found that Preeclampsia decrease the incidence of RDS. Also we found that there were increased number of neonates in whom their mother received prenatal steroid in patient received NCPAP only (group A) than those received NCPAP with surfactant (group B) (30% vs.6%) with p value < 0.005. This may explain good results observed in patient with NCPAP only as their lungs were more mature and so need less respiratory support to improve. This is in agreement with (Jobe et al., 1993) & (Crowley. 2002), [10] and [11]. In this study we noticed that the respiratory rate in cases with RDS after 24 hours of initial stabilization is significantly lower in both groups; patient received NCPAP only (group A) and patient received surfactant with NCPAP (group B). Also there were significantly lower mean values of respiratory score at 24 hours after initial stabilization in both groups A and B.The results of this study were in agreement with Gamil et al., (2007), [12] which showed that the earlier the use of NCPAP, the more significant lowering of the respiratory rate as evidenced by a significantly lower mean respiratory rate at 24 hours of initial stabilization in immediate and early (before 6 hours) CPAP groups compared to non CPAP group. This result can be explained by that CPAP works in a number of ways: it reduces the of upper airway collapse, stabilize the chest wall, decreases upper airway resistance, reduce work of breathing [13]. It increases the pharyngeal cross-sectional area and decreases genioglossal activity by its mechanical effects of raising the intraluminal upper airway pressure above the positive critical transmural pressure of the pharynx or hypopharynx [14]. It has also been noted that with the passive distention in the upper airway there is a concomitant reduction in phasic electrical activity of the genioglossal muscles [15]. CPAP also alters the shape of the diaphragm and increases its activity, improves lung compliance, this allows a greater tidal volume for a given negative pressure. CPAP also seems to conserve surfactant on the alveolar surface, and therefore may have a synergistic effect [16]. Finally it will reduce the respiratory rate via the Hering-Breuer reflex [17]. In this study we noticed improvement in all parameters of blood gases (PH, PO2, PCO2, HCO3) among the studied groups of patients (group A & B) at 1 hour and at 4 hours after admission with no significant difference between both groups. These results matched the results of the study done by Reininger et al. (2005), [18] as when they investigated infants 29 to 35 weeks’ gestation with mild to moderate RDS in which they were randomized into 2 groups (NCPAP group and NCPAP plus surfactant group), they found that there was no difference between 2 groups in median alveolar-to-arterial oxygen (a/A) ratio (p value 0.08) or mean PaCO2 when ABG was done within 4 hours from randomization. These results were not in agreement with the study done by Verder et al., (1994), [19] which showed that there was significant improvement in mean ± SD of PO2 in surfactant group than CPAP group (p value < 0.01). They reported that combination of early NCPAP with surfactant would improve RDS as indicated by increase in oxygen tension ratio and decrease in transcutaneous partial pressure of carbon dioxide. Respiratory distress in preterm infants is associated with delayed absorption of fetal lung water owing to defective sodium transport mechanism [20]. CPAP enhances lung expansion and fluid clearance, and if applied early, CPAP will aid in the transition phase and improve oxygenation [21]. It is the positive end expiratory pressure that will help form and maintain FRC. Starting CPAP immediately after birth in spontaneously breathing extremely preterm infants is crucial because the non-compliant lung will otherwise collapse and high positive pressure is more likely to be required to open the lung with the subsequent risk of injuring the lung [22]. Also when CPAP increase FRC, this will result in improving PaO2, decreasing airway resistance, reducing obstructive apnea. CPAP increases the transpulmonary pressure which results in a greater thoracic gas volume and FRC [21]. This would increase the surface area for gas exchange and decreasing intrapulmonary shunt. Improved gas exchange after recruitment of alveoli with surfactant and CPAP can allow for lower FiO2 thereby reducing oxygen toxicity. Maintaining an adequate FRC as soon after birth as possible will result in stabilization of air spaces, prevent the formation of atelectasis and promote the release of surfactant stores [23]. CPAP improves oxygenation without increasing peripheral arterial carbon dioxide tension (PaCO2) [24]. A significant improvement in oxygenation is a consistent finding after administration of surfactant. The improvement in oxygenation is due to the ability of surfactant to stabilize the alveoli on expiration and allow an increase in FRC and possibly via an effect on pulmonary blood flow. Walmrath et al., (1996), [25] showed that bronchoscopic application of a natural surfactant resulted in an ‘immediate, impressive, and highly significant improvement of arterial oxygenation in all patients, due to a marked reduction of shunt flow. In this study there was marked improvement in CXR among the studied groups A & B of patients at 6 hour than on admission with no significant difference between both groups (table 4) as follow: Patient improved from 2nd to 1st degree were 30% vs 22%, from 3rd to 2nd degree were 38 % vs 24%, from 3rd to 1st degree 28% vs 34% in treatment groups (A) vs control group (B). Patient had 4th degree in CXR improved to 2nd degree were 4% in group (A) vs 16% in group B and only 4% improved from 4th degree to 3rd degree in group (B). This can be explained by that the effects of surfactant therapy on RDS can be divided into pulmonary, cardiac, and radiologic. The immediate pulmonary effects include rapid improvement in oxygenation accompanied by increasing functional residual capacity, followed by a variable increase in lung compliance. The radiologic changes reflect the recruitment of lung volume and decrease in atelectasis after surfactant treatment [26]. TCUS on the 2nd day on group (A): 19 (38%) normal, 29 (58%) 2nd degree IV Hemorrhage & no one have 3rd degree intraventricular Hemorrhage compared to 24 (48%) normal, 23 (46%) 2nd degree intraventricular Hemorrhage & 1 (2%) have 3rd degree intraventricular Hemorrhage in group (B). TCUS on the 7th day on group (A) vs group (B): 48% vs 62% became normal, 4% vs 2% became 1st degree intraventricular hemorrhage, 26% vs 18% became 2nd degree intraventricular & 14% vs 8% became 3rd degree intraventricular hemorrhage & 8% vs 10% not done. The percentage of improvement in the 2 groups was as follow: 14% improved in group (A) vs. 16% in group (B), 64% the same in group (A) vs. 68% in group (B), 14% worse in group (A) vs. 6% in group (B) with no significant difference between both groups. So as we see there was reduction in number of patient with 2nd & 3rd degree intraventricular hemorrhage on day 7 from 26%, 14% in NCPAP group to 18%, 8% in surfactant group. our results matched the results of the study done by Verder et al., (1994), [21] who showed that there was marked reduction in patient with grade 3 and 4 intracranial hemorrhage from 15% in CPAP group to 9% in surfactant group on day 7 and also in total patient survive or died by grade 3 and 4 intracranial hemorrhage from 27% in CPAP group to 11% in surfactant group. As well as the results of the study done by Finer et al., (2010), [27] that showed reduction in percentage of 3rd & 4th degree intraventricular hemorrhage from 14.3% in CPAP group to 11.5% in surfactant group. Our results also matched with study done by Sandri et al., (2010), [28] that showed slight reduction in 3rd & 4th intraventricular hemorrhage from 7.8% in CPAP group to 5.7% in surfactant group. In contrast with study done by Rojas et al., (2009), [29] in which there was no difference between 2 groups: 2% in CPAP vs 1% in surfactant group and the study done by Dunn et al, (2011), [30] the percentage with any type of intraventricular hemorrhage was 21.6% in NCPAP group versus 20.9% in surfactant group. As we know that one of the recorded side effects of surfactant treatment include increased cerebral blood flow velocity, which, due to the lack of cerebral vascular autoregulation in many sick preterm infants. Various authors have reported transient changes in blood pressure, cerebral blood flow velocity, cerebral oxy-haemoglobin concentration and change in cerebral activity on EEG which can lead to intraventricular haemorrhage or periventricular leukomalacia [31]. Cerebral blood flow response is similar to the acute increases or decreases in PCO2 associated with surfactant administration [32]. Our results can be explained by that mothers of some of our infants received antenatal corticosteroids which induce fetal lung maturation and lead to maturation and involution of the fragile germinal matrix vasculature, definitely reduce the overall incidence and severity of germinal matrix hemorrhage /intra-ventricular hemorrhage (GMH/IVH), [33] and [34]. Additionally complications of respiratory distress syndrome e.g., pneumothorax, bronchopulmonary dysplasia, are associated with a high risk of hemodynamic disturbances. Treatment with surfactant is the single most important advance in the management of lung immaturity. Collective data from the Cochrane Database suggests that intratracheal surfactant, either natural or synthetic, administered prophylactically in the delivery room is most efficacious [35]. Cochrane review has reported that intramuscular betamethasone (12 mg given twice at 24 hour interval) given in 24-34 weeks of gestation results in reduction in neonatal mortality, RDS, cerebro-ventricular hemorrhage (by 40%), and systemic infections in the first 48 hours of life [36] and [37]. In this study we found that the most common co-morbidity is neonatal sepsis which had the same percentage in both groups A & B (20%) (table 5), followed by PDA that persist in 4% in group (A) vs 14% in group (B). Intra ventricular hemorrhage was 14% in group (A) vs 6% in group (B), number of cases with intrapulmonary hemorrhage was equal 4% in both groups and number of cases with necrotizing enterocolitis and neonatal sepsis was 2% vs 4% in group (A) compared to group (B). There was only one case in group (B) with neonatal sepsis, liver cirrhosis and hepatitis C virus, one case with pneumothorax, and one case complicated with Post Hemorrhagic hydrocephalus in group (A). The number of cases recorded without complications were 44% in group (A) vs 56% in group (B). Increase percentage of neonatal sepsis in our patients could be explained by that they are from low socioeconomic status and receive poor antenatal care. We face problems of inappropriate nurse: patient ratios and overcrowding. Also in surfactant group the use of endotracheal tube is an invasive maneuver which leads to neonatal sepsis. This is in agreement with (Femitha et al., 2012), [38]. Also most cases of group B were extremely preterm ≤ 29 week (54%) and VLBW (68% in group A vs. 76% in group B), these factors increase the vulnerability of these neonates to infection. Regarding cases with PDA in our study, were 4% in group A vs. 14% in group B, this can be explained by that exogenous surfactant therapy lead to a rapid drop in pulmonary vascular resistance and increase left-to-right shunt in preterm infants with RDS, increase in ductal shunt velocity and increased pulmonary blood flow which lead to the persistently opened PDA. This is in agreement with (Clyman, 2006), [39]. The improved oxygenation may increase PDA closure rates [40]. This is not in agreement of our results as PDA persist in 14% in cases of group B who received surfactant with improved oxygenation. The risk of pulmonary hemorrhage increases with surfactant therapy and hemorrhagic pulmonary edema is the source of blood in many cases and is associated with significant ductal shunting and high pulmonary blood flow associated with surfactant therapy due to improvement in the pulmonary vascular resistance and left to right shunting occurs, [2]. However our cases had intrapulmonary hemorrhage in 4% of cases of both groups with no difference between both groups related to surfactant therapy. Surfactant replacement therapy may lead to barotraumas due to increase in lung compliance and failure to bring down ventilator settings with increasing tidal volume which may result in pneumothorax (Koch et al., 2010) [41], (David et al., 2012) [42], and (Walsh et al., 2013), [43] found no significant differences between both groups in the rates of pneumothorax and intraventricular hemorrhage. Our results matched the results done by Verder et al., (1999), [44] and Femitha et al., (2012), [38] who reported that the most common co-morbidity was sepsis (in 43.5%), followed by PDA (3.9%) and pulmonary hemorrhage (3.9%) then NEC (1.9%) and pneumothorax (1%). Imani et al., (2013), [11] show same percentage of patients with intrapulmonary hemorrhage 7.5% and pneumothorax 5%.However, our study was not in agreement with the study done by Koti et al., (2010), [45] they found increased number of patient with PDA from 12% in CPAP group to 30% in INSURE method and increased percentage of sepsis and pneumonia in patient received surfactant than in patient received CPAP only (30% vs 4.8 %), increase in number of patient develop 3rd degree Intrventricular Hemorrhage in INSURE method from 0% to 12% in CPAP group but pneumothorax decease in INSURE method from 4.7% in CPAP group to 0% in INSURE group. Imani et al., (2013), [11] reported decrease in cases of PDA from 15% in CPAP group to 5% in surfactant group. In this study we found that there was no significant differences in duration of hospital stay between both groups A & B as Mean ± SD of hospital stay was (20.18 ± 15.35 vs. 20.56 ± 17.12 days, P-value: 0.907) between group (A) & group (B) respectively. This is in agreement with Reininger et al. (2005), [18], Rojas et al., (2009), [29] and Saianda et al., (2010), [46]. The length of hospital stay increased as the gestational age and birth weight decreased [34]. Although the cost of surfactant is quite high, it decreases the duration of ventilation and NICU stay. It is actually quite cost effective as it helps in providing a healthy productive life to a preterm baby with low probability of abnormal neurological outcome [37] and [47].In this study we found that there was reduction in number of patients connected to MV from 42% in CPAP group (A) to 38% in surfactant group (B) with no significant difference between both groups, with patient connected in the 1st day of life 9(18%) in group (A) vs. 7(14%) in group (B). This is in agreement with the studies done by Reininger et al., (2005), [18], Rojas et al., (2009), [29] and Imani et al., (2013), [9]. However our results were not in agreement with Verder et al., (1994), [19], Verder et al., (1999), [44] and Dani et al., (2004), [3]. Saianda et al., (2010), [46] found that there was marked reduction in connection to MV from 28% in treatment group to 16% in control group with p value 0.05. In our study the duration of respiratory assistance among both groups was as follow: the duration of oxygen therapy was significantly shorter in group B than in group A and also the duration of mechanical ventilation was significantly shorter in group B than in group A. This could be explained by that surfactant replacement therapy result in maintaining a gas reservoir at the end of expiration (FRC), maintaining optimal compliance of the lung, enhancing liquid efflux from the alveoli, lowering work of breathing and oxygen consumption, hence lowering the need for oxygen therapy and mechanical ventilation. This is in agreement with Rojas et al., (2009), [29] and (Sen. 2010), [40]. In contrast to our study, Reininger et al., (2005), [18] and Dunn et al., (2011), [30] reported that the duration of O2 therapy was similar in both groups as well as the duration of MV was not significantly different in both groups. The mortality rate in this study was significantly different between both groups (12% in group (A) vs 4% in group (B), p value 0.001) (table 6). This is in agreement with the study done by Saianda et al., (2010), [46] and Imani et al., (2013), [9]. However our result doesn't match the results of Rojas et al., (2009), [29] in which there were no significant difference between 2 groups as mortality rate was the same 9%, and Koti et al., (2010), [45] who showed decrease number of mortality to 2% only in CPAP group. Femitha et al., (2012), [38] reported neonatal mortality 21.8 % in CPAP group vs 30.3% in surfactant group, and in the multicenter, randomized SUPPORT (Surfactant, Positive Pressure, and Oxygenation Randomized Trial), (2011) there were no significant difference between both groups regarding the mortality rate. We believe that the improved outcomes observed among infants who were randomly assigned to the treatment group were attributable to the addition of very early surfactant with standardized PPV. Conclusions: This study demonstrates that preterm neonates born at 28 to 32 weeks’ gestation supported with NCPAP only compared with those received surfactant therapy using INSURE method (intubation- surfactant administration followed by extubation to NCPAP), there were an improvement of clinical sign in the form of improvement of tachypnea, oxygenation, acid base disturbance, slight reduction in need for mechanical ventilation, duration of stay on MV, reduction of air leak syndrome, occurrence of 3rd degree intra-ventricular hemorrhage or persistence of PDA. There were significant difference in imaging study including improvement in chest X ray, also duration of oxygen therapy and so development of broncho-pulmonary dysplasia, duration of hospital stay, and mortality, thus reduced costs associated with the administration of surfactant. Using early NCPAP leads to reduction in the number of infants who were intubated and receive surfactant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML