-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(1): 29-35
doi:10.5923/j.ajmms.20170701.07

Seropositivity of IgG Antibodies against Measles Virus in Unvaccinated Children Population of Emohua in Rivers State, Nigeria
Iheanyi Omezuruike Okonko, Mercy Elenwo
Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria
Correspondence to: Iheanyi Omezuruike Okonko, Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Immunoglobulin G (IgG) antibodies determination is of countless significance to evaluate vaccination and immunization programmes for vaccine-preventable diseases. The low seropositivity of vulnerable populations has previously led to the eradication of indigenous measles elsewhere and this would help in the eradication of indigenous measles in Nigeria. This study was designed to determine the seropositivity of measles virus IgG antibodies in unvaccinated children population of Emohua in Rivers State, Nigeria. Samples were collected from 91 unvaccinated children (1month - 10 years) and evaluated for seropositivity of measles virus-specific IgG antibodies using standard ELISA. Socio-demographic characteristics of the children were obtained using structured questionnaire. Chi-square and Fisher's exact test were used to compare proportions at p<0.05 significant level. The overall seropositivity of measles virus-specific IgG antibodies was 58.2%. No clinically or statistically significant difference (p>0.05) in seropositivity rate of measles virus-specific IgG antibodies was found in males and their female counterparts (58.3 vs. 58.1%). However, age, education and previous measles exposure (p>0.05) were statistically related to seropositivity of measles virus-specific IgG antibodies. Also, group-specific negativity was low (16.7 – 56.5%) though no statistical relationship whatsoever, was observed for any of the socio-demographic data. This further queried if truly, measles vaccination is administered widely in Nigeria and thus able to prevent severe reinfection with the wild type. This study further confirms that measles remains endemic in Emohua, Rivers State, Nigeria. It is therefore recommended that immunization be given to these children.
Keywords: Antibodies, Immunization, Measles IgG, Measles virus, Seropositivity, Vaccination
Cite this paper: Iheanyi Omezuruike Okonko, Mercy Elenwo, Seropositivity of IgG Antibodies against Measles Virus in Unvaccinated Children Population of Emohua in Rivers State, Nigeria, American Journal of Medicine and Medical Sciences, Vol. 7 No. 1, 2017, pp. 29-35. doi: 10.5923/j.ajmms.20170701.07.
1. Introduction
- Measles is a major vaccine-preventable infectious disease and one of the greatest transmissible and highly infectious diseases of children in the tropics and its resurgence offers worthy instance of the fast spread of the virus [1, 2]. It is one of the main infant killer diseases leading to an extensive epidemics in developing nations notwithstanding the obtainability of an effective and safe vaccine [3-6]. Measles is so infectious that any child who is slightly exposed to it and is not immune will possibly get infected [7]. Measles is worldwide-reaching nonetheless it is prevalent in emerging nations where austere illness and high death are linked to underlie poverty, malnourishment, indiscriminate vaccination service and poor health systems [6, 8-11]. In 1980, measles caused an estimated deaths of 2.6 million people preceding the extensive measles vaccination.In 1990s, it caused estimated one million deaths globally [6, 12]. In 2000, it caused an estimated 546,800 deaths [13]. In 2008, 164,000 deaths occurred mostly in the low-income nations [6, 11] and in 2014, about 114, 900 deaths (mostly children under five years of age) [13]. This decrease in measles mortality is attributed to the enhanced vaccination activities [13].Several factors (such as primary vaccine failure, poor state of vaccine storage, low potency of the vaccines, numerous importations and inappropriate handling of vaccines during immunization) has been responsible for measles resurgence in developing nations [2, 12, 14-25]. This study seeks to evaluate the seropositivity of measles virus-specific IgG antibodies in unvaccinated children population of Emohua in Rivers State, Nigeria. The study also seeks to evaluate the risk factors of seropositivity for measles virus IgG antibodies among unvaccinated children and assess their susceptibility to the measles virus.
2. Methods
- Study areaThis study employed unvaccinated children presenting at Rumuewhor Primary Health Centre, in Emohua Local Government Area of Rivers State, Nigeria. Rumuewhor community comprises 4 villages (Rumuewhor, Rumuodugo I, Rumuodugo 11 and Eruewku). It has an estimated population of 18,722. It is a rural area where majority of the indigenes are farmers. The major occupation of people in that community is farming from which they derive so much joy and satisfaction. The lifestyle of these people (in certain aspects such as hygiene, nutrition, etc.) is poor as they do not have value for medical treatment and care, but prefer their traditional herbal methods. However, these could be attributed to die hard beliefs and ignorance. There is a primary health centre owned and run by the state government which happens to be the only hospital in that community. This health centre carries out free medical care for adults and children which includes; immunization, maternal and child care and other health services. However, these free services are not well utilized by people in the community and as such there is a high level of unvaccinated children and adults. The health care services are free for adults and children, services includes immunization, maternal and child care services, nutritional rehabilitation, growth monitoring. The villagers believe immunization would lead to death of their children because of the fever associated with it so they refuse to vaccinate their children.Study populationNinety-one (91) unvaccinated children attending the Rumuewhor Primary Health Centre in Emohua, Rivers State, Nigeria were enlisted after giving a written/verbal informed consent. Unwilling children were excluded. Socio-demographic characteristics of the children were obtained using structured questionnaire (Table 1). The sample size was determined as described by Macfarlane [26], Naing et al. [27] and Awando et al. [28]. Of the 91 children studied, 23(25.3%) was aged 0–8 months, 50 (54.9%) was aged 9 months to 5 years and 18 (19.8%) was children aged 6–10 years. Sample collection and plasma preparationA 2 mL blood sample was aseptically collected from each unvaccinated children in anticoagulant containers containing ethylenediaminetetraacetic acid (EDTA). Samples were conveyed to the Medical Microbiology Laboratory of the Department of Microbiology, University of Port Harcourt, Nigeria in a cold chain. The blood samples were centrifuged and the plasma aspirated into eppendorf tubes and kept at -20°C. Methods were in agreement with the ethical standards of the Nigerian National Code for Health Research Ethics and the Declaration of Helsinki (October 2008 revision).Serological testingThe samples were analyzed for measles virus-specific IgG antibodies using immunoglobulin G measles enzyme-linked immunosorbent assay kits (DIA.PRO Diagnostic Bioprobes, Milano, Italy). The serologic test and interpretation was carried out according to the manufacturer’s specifications. Data Analysis Results were presented in proportions. We employed Chi-square test and Fisher's exact test to evaluate variances amid groups at p ≤ 0.05 significance. We used <1.0 as negative and ≥1.0 as positive in order to get valid analysis.
3. Results
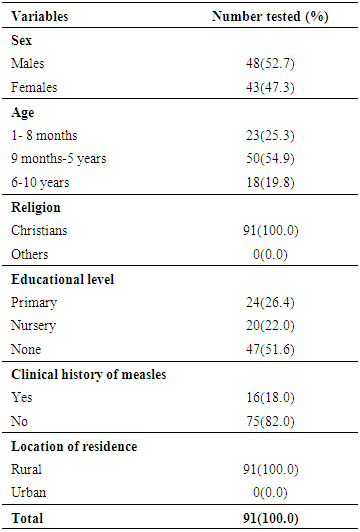
- Children characteristicsSocio-demographic characteristics of the unvaccinated children and information on the possible risk factors are shown in Table 1. More males than females (52.7% vs. 47.3%) partook in the study. This did not differ by sex (p>0.05). The ages of the unvaccinated children ranged from 1 month - 10 years. Age group 9 months to 5 years had the highest frequency [53(54.9%)] in comparison to the other groups. This was followed by age group 1 month – 8 months [23(25.3%)] while age group 6-10 years was [18(19.8%)]. They differ significantly (p<0.05) in age [Table 1]. Twenty-four (26.4%) of the children were in their primary level of education and 20(22.0%) in play group/nursery/kindergarten education while 47(51.6%) were not in any educational level (Table 1). All (100.0%) of the participants were Christians. Sixteen (18.0%) of the 91 children have had measles infection in the past while 75(82.0%) had no previous exposure to measles infection (Table 1).
|
|
|
|
4. Discussion
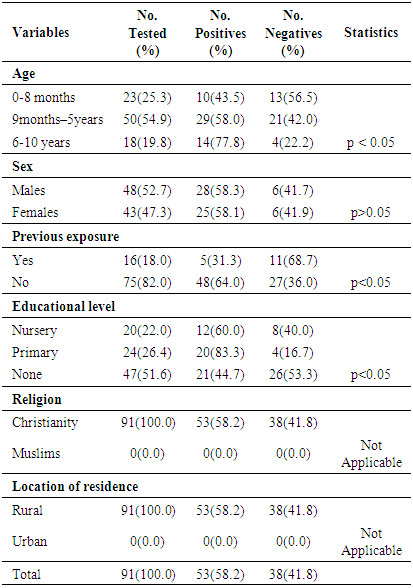
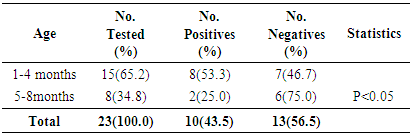
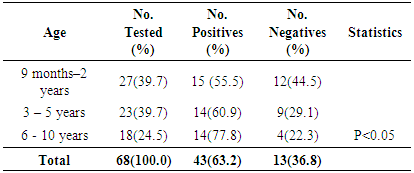
- Measles morbidity and mortality remained a significant public health concern in Africa. Furthermore, the current measles resurgence is an essential pointer of how short-lived immunity improvements and development can be [11, 29]. In order to reduce measles transmission, higher population immunity must be attained and upheld [29]. This study seeks to evaluate the seropositivity of measles virus-specific IgG antibodies in unvaccinated children population (ages 1 month to 10 years) of Emohua in Rivers State, Nigeria. The study also seeks to evaluate the risk factors of seropositivity for measles virus-specific IgG antibodies among unvaccinated children and assess their susceptibility to the measles virus. This is the first documented seropositivity of measles virus-specific IgG antibodies amongst unvaccinated children population of Emohua Rivers State, Nigeria. Among all the 91 children screened for measles virus-specific IgG antibodies, 53(58.2%) had measles virus-specific IgG antibodies (S/Co ratio ≥1.0 international standard) while the remaining 38(41.8%) had measles virus-specific IgG antibodies (S/Co ration <1.0 international standard). The 58.2% seropositivity of measles virus-specific IgG antibodies is lower than the 75.8% reported by Rafiei et al. [30] in Tehran; the 76.0% reported by Domínguez et al. [31] and Plans et al. [32] among individuals aged <25 years; the 89.0% reported by Domínguez et al. [31] and Plans et al. [32] among non-vaccinated individuals; the 98.6% reported by Condorelli et al. [33]; the 98.3% reported by Domínguez et al. [34]; the 97.6% reported by Shilpi et al. [35]; the 98.5% reported by Plans et al. [32] in related studies. However, the 58.2% seropositivity of measles virus-specific IgG antibodies reported here is comparable to the 50.0% reported by Plans et al. [32] among children aged ≤15 months in Catalonia. Seropositivity of measles virus-specific IgG antibodies were prevalent among children eligible for vaccination; however, only 55.5% of vaccination-age children had measles virus-specific antibodies [29]. Low (25.0%) seropositivity of measles virus-specific IgG antibodies was found among unvaccinated children aged 0-8 months, leaving this children vulnerable to indigenous measles virus transmission [29]. The cause of this low seropositivity is not clear [29].Of the 23 unvaccinated children (aged 0-8 months), 10 (43.5%) tested positive for measles virus-specific IgG antibodies. Higher seropositivity was observed unvaccinated children (aged 1-4 months) while unvaccinated children (aged 5-8 months) had lower seropositivity (25.0%) and none of the children in this age group had evidence of measles vaccination [32, 36]. Low waning maternal antibody protection, which led to an increased period of vulnerability to natural measles infection before vaccination age (9 months), has been reported in developing nations in past 20 years [29, 37-41]. In a recent study in Bangladesh, only 50% of infants aged 1-3 months had protective antibodies and infants aged >3-9 months had no protection at all, similar to this present findings and other previous studies [29, 37-42]. Also, 55.5% (15/27) seropositivity was reported among unvaccinated children aged 9 months to 2 years. Increase in seropositivity of measles virus-specific IgG antibodies was found among older unvaccinated children, 60.9% (14/23) for those aged 3-5 years and 77.8% (14/18) for children aged ≥6 years. This may be attributed to natural exposure to measles virus infection since they were not vaccinated. This is also related to the findings of Itolh et al. [43], who reported a drop in measles antibody as the children grows older. The study showed age-related seropositivity (p<0.05) of measles virus-specific IgG antibodies among the various age groups. Generally, higher seropositivity of measles virus-specific IgG antibodies was observed in age group 9 months -5 years (54.9%). This was followed by age group 0 – 8 months (25.3%) while ages 6 - 10 years had the least seropositivity (19.8%). The seropositivity of measles virus-specific IgG antibodies increased with increasing age, then decreased in older children. This disagrees with Rafiei et al. [30] who reported that no substantial age association with seropositivity of measles virus-specific IgG antibodies. This corroborates the findings of Shilpi et al. [35].The study showed no sex-related (58.3% vs. 58.1%, p>0.005) connection with the seropositivity of measles virus-specific IgG antibodies. Klingele et al. [44], Ogundiji et al. [45] and Rafiei et al. [30] also reported no significant sex-related connection. This finding deviates from Black [46], Chen [47] and Akyala et al. [48] who reported significant sex-related association. In this study, educational level (p<0.05) was significantly related to seropositivity of measles virus-specific IgG antibodies. Seropositivity of measles virus-specific IgG antibodies was higher among children in their primary level of education (83.3%), followed by those in their pre-nursery/nursery education (60.0%) while children yet to commence education in life had the least seropositivity of 44.7%. Measles virus infection remains an important public health issue particularly in developing countries especially in African and Asia. Morbidity and mortality attributed to measles virus is increasing particularly within infants at the age of 1-5 years without measles vaccine. Of the 16(18.0%) children with previous history of exposure to measles virus infection; 5(31.3%) tested positive for measles virus-specific IgG antibodies and 48(64.0%) with no such history also tested positive. There was a significant difference (p<0.05) in the seropositivity of measles virus IgG-specific IgG antibodies and previous exposure to measles infection. This is related to their immunity system. The seropositivity of measles virus-specific IgG antibodies is high among children who were infected with measles virus and still have their antibody acquired from their mother but is low in children of 6 years and above who had not been exposed to measles infection and had lost the antibody acquired from their mothers. The outcome of this detectable pre-vaccination measles antibody across the age group is that most of the children had no measles vaccine but were exposed to measles virus and some still have the immunity acquired from their mothers (placenta transfer and breast milk [2, 48].
5. Conclusions
- This study further confirms that measles remains endemic in Emohua, Rivers State, Nigeria. The results presented in this study were still similar to the results of earlier studies in Nigeria and outside. The seropositivity of measles virus-specific IgG antibodies among unvaccinated children population of Emohua in Rivers State, Nigeria is slightly low (58.2%), signifying that a greater number of children might not be protected against measles. It is therefore recommended that immunization be given to these children. Consequently, complementary seroepidemiological studies to point the changing aspects of measles virus-specific IgG antibodies in other areas of Rivers State, Nigeria should be carried out to improve comprehending the gap of susceptibility of children and the immunity profile of older age groups all over the country.
ACKNOWLEDGEMENTS
- The authors sincerely acknowledge the Management and staff of Rumuewhor Primary Health Centre, in Emohua Local Government Area of Rivers State, Nigeria for their cooperation and support. We also appreciate all the children and parents for their consent, cooperation and participation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML