-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2016; 6(3): 73-86
doi:10.5923/j.ajmms.20160603.03

Nephrotoxic Effects of Abamectin and Calotropis procera Latex and Leaf Extract in Male Albino Rats
Hanaa I. Fahim1, Osama M. Ahmed1, Magdy W. Boules2, Heba Y. Ahmed2
1Physiology Division, Department of Zoology, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt
2Rodents Division, Department of Harmful Animals, Plant Protection Research Institute, Agriculture Research Center, Egypt
Correspondence to: Osama M. Ahmed, Physiology Division, Department of Zoology, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The current study aims to assess the toxic effect of abamectin and latex and ethanolic extract of leaves of Calotropisprocera administrations on kidney function and histological integrity in male albino rats. LD50 of latex was determined through oral administration of latex in gradual increasing doses (0.4, 0.8, 1.2, 1.6, 2, 2.4, 2.8, 3.2 ml/ kg b. wt) for 8 groups each of 5 male albino mice. After 72 hours of administration, the number of dead animals in each group and LD50 was calculated for mice and converted to its equivalent in rats. The albino rats used in this study were allocated into 7 groups. Group 1 was orally administered 1% carboxy methyl cellulose (CMC) as a vehicle for 4 weeks and 8 weeks and was regarded as control group. The second and third groups were orally treated with 1/20 (66 µl/kg b. wt) and 1/10 (132 µl/kg b. wt) of LD50 of Calotropisprocera latex respectively for 4 weeks and 8 weeks. The fourth and fifth groups were orally administered 1/20 (4.78 mg/kg b. wt) and 1/10 (9.56 mg/kg b. wt) of LD50 of ethanolic extract of C.procera leaves respectively for 4 weeks and 8 weeks. The sixth and seventh groups was orally administered with 1/20 (0.44 mg /kg b. wt) and 1/10 (0.87 mg/kg b. wt) of LD50 of abamectin (vertimec 1.8% EC) respectively for 4 weeks and 8 weeks. C.procera latex and ethanolic extract as well as ABM produced a marked elevation of serum urea, uric acid and creatinine level at the two tested periods in a dose dependent manner. The increase in lipid peroxiation and depletion in glutathione level and glutathione peroxidase, glutathione-S-transferase and superoxide dismutase activities occurred in kidney of all treated rats. Treatments with the plant latex and leaves ethanolic extract as well as ABM induced a significant increase in serum tumor necrosis factor -α and decrease in serum interleukin-4 and these changes were dose dependent. Alterations in the normal histological structure and integrity of kidney were observed after treatments and degree of lesions became more deleterious as the time progressed from 4 to 8 weeks. In conclusion, the findings of this study indicated that C.procera latex and ethanolic extract of leaves could induce marked toxicity in kidney and these toxic effects may be more or less similar to those of abamectin. This nephrotoxicity may be mediated via stimulation of oxidative stress and suppression and antioxidant defense system. However, further studies are required in future to assess the toxic effects of various constituents ofC.procera latex and leaves ethanolic extract.
Keywords: Calotropisprocera, Latex, Ethanolic leaves extract, Abamectin, Kidney, Toxicity
Cite this paper: Hanaa I. Fahim, Osama M. Ahmed, Magdy W. Boules, Heba Y. Ahmed, Nephrotoxic Effects of Abamectin and Calotropis procera Latex and Leaf Extract in Male Albino Rats, American Journal of Medicine and Medical Sciences, Vol. 6 No. 3, 2016, pp. 73-86. doi: 10.5923/j.ajmms.20160603.03.
Article Outline
1. Introduction
- Many natural products have been suggested as alternatives against conventional chemical pest control. Plant extracts have been used as pesticides by humans since before the time of the ancient romans, a practice that continues to the present time with many of the plant species known to have pesticidal properties [1]. The use of toxic plants is especially prevalent in the developing countries, where plants grown locally are cheaper than the synthetic chemical pesticides [2]. Many plant substances and constituents have been reported to have rodenticidal effect [3-5].Calotropis (C.), is a genus that have 6 species of shrubs or small trees, distributed in tropical and subtropical Africa, Asia and America. Two species namely C. procera and C. gigantae are found in India which closely resembled to each other in structure and in functional uses [6]. It was revealed that C. procera contains various chemicals which are useful for various activities [7, 8]. The entire plant has been reported to contain flavonoids, alkaloids, sterols, triterpenoids, cardiac glycosides and usharin [9]. In an earlier study, various medicinal properties such as laxative, anti-inflammatory, anthelmintic, purgative, and diuretic have been documented [10]. Different parts of C. procera and its latex have shown analgesic, anti-bacterial and wound healing properties in traditional medicine [11, 12]. The previous pharmacological studies on C. procera include reports of its anticancer, antifungal and insecticidal activity [13, 14].Despite these uses, C. procera poses varying toxic effects in animals and humans through touch, air borne allergies, and consumption in livestock. Vadlapudi and Naidu [15] revealed that the plant is also known for its toxic properties that include iridocyclites, dermatitis and acts like a poison and produces lethal effects. Toxicity of C. procera is reported in sheep in the form of diarrhea and anorexia. Consumption of this plant leads to severe poisoning to livestock as well as man. Incidental ingestion of fresh C. procera leaves has been suggested as toxic to many ruminants by several farmers especially from the Brazilian semi-arid region. These observations are supported by some studies that have reported toxic effects promoted by C. procera latex and leaves [16-18]. The latex of C. procera contains several alkaloids (such as calotropin, calcilin, catotoxin and gigantin) which are caustic and considered poisonous in nature [19].Abamectin (ABM), the non-proprietary name assigned to avermectin B1, is a mixture of two components, with the major component avermectin B1a 80% of the mixture, and the minor component avermectin B1b, 20% of the mixture, differing by a single methylene group [20]. The two components, B1a and B1b, have similar biological and toxicological properties [21, 22]. As indicated by Kolar et al. [23], ABM has been used in several countries as a pest control agent in livestock and as an active substance of insecticides and nematicides for agricultural use. ABM may be valuable in agriculture; it may be highly toxic to mammals [24].Therefore, this study aims to verify the toxic effect of latex and ethanolic extract of leaves of C. procera compared with the biocide ABM on kidney of rats.
2. Materials and Methods
2.1. Experimental Animals
- Male albino mice (8-9 weeks of age) weighing 20-25 g and male albino rats (8-10 weeks of age) weighing 120-150g were used as experimental animals in this investigation. They were obtained from the Animal House of Research Institute of Ophthalmology, Giza, Egypt. Animals were supplied daily standard pellet diet and were given water ad libitum. The animals were housed in polypropylene cages with good aerated stainless steel in Animal House of Zoology Department, Faculty of Science, Beni-Suef University, Egypt at temperature 20-25°C and 12-hours daily light dark cycles. The animals were kept for 2 weeks under observation before the onset of the experiment to exclude any intercurrent infection. All animal procedures are in accordance with the recommendation of the Experimental Animals Ethics Committee of Faculty of Science, Beni-Suef University. All efforts were done to decrease the suffering of animals to a minimum.
2.2. Plant Materials
- The leaves and latex of C. procera were obtained from East desert of Beni-Suef Governorate. The plant was authenticated by Dr. Walaa Azmy Hasan, lecturer of plant taxonomy, Department of Botany, Faculty of Science, Beni-Suef University.
2.2.1. Leaves Collection and Extract Preparation
- Only mature leaves without signs of lesions were used. The leaves of Calotropis procera were extracted by ethanol according to Freedman et al. [26]. Leaves were air dried at room temperature then ground into fine powder using an electrical grinder. 500 Grams of the powder were suspended in one liter of ethanol 95% for 72 hours then filtered and the filtrate was evaporated by rotary evaporator at 40-50°C at Faculty of pharmacy, Beni-Suef university. The extract was kept in refrigerator at -30°C until use.
2.2.2. Latex Collection
- Fresh latex was obtained by breaking the leaf stock and allowing the latex to flow into a glass beaker. It was freshly prepared before injection.2.2.2.1. Acute Toxicity and Determination of Oral LD50 of C. procera LatexLD50 of the latex was determined as described by Afifi et al. [26] and Ahmed et al. [27]. Eight groups, each of 5 mice were used. Mice were orally administered the latex by gastric tube in gradual increasing doses (0.4, 0.8, 1.2, 1.6, 2, 2.4, 2.8, 3.2 mg/ kg b. wt). After 72 hours of administration, the number of dead animals in each group, the main dead animals in two successive doses (z) and the constant factor between two successive doses (d) were recorded and LD50 was calculated as follow:LD50 = the biggest dose which kill all animals - ∑(z.d)/nWhere n: number of animals in groups = 5 animals in each group. LD50 of albino rats was calculated from that of mice by using the conversion table of Paget and Barnes [28].
2.3. Pesticide
- Abamectin (1.8% EC): is a mixture of 80% avermectin B1a and maximum of avermectin B1b used as an acaricide. It was obtained from Synganta Agro. Co., Switzerland.
2.4. Experimental Design
- The male albino rats were allocated into 7 groups. Group 1 was regarded as control and was administered 1% carboxymethylcellulose (CMC) by oral gavage for 4 weeks and 8 weeks. Group 2 was orally administered 1/20 of LD50 of C. procera latex (66 µl/kg b. wt), dissolved in 1% CMC, for 4 weeks and 8 weeks. Group 3 was orally administered 1/10 of LD50 of C. procera latex (132 µl/kg b. wt), dissolved in 1% CMC, for 4 weeks and 8 weeks. Group 4 was orally administered 1/20 of LD50 of ethanolic extract of C. procera leaves (4.78 mg/kg b. wt) dissolved in 1% CMC for 4 weeks and 8 weeks. LD50 of ethanolic extract of C. procera leaves was 95.52 mg/kg b. wt [29]. Group 5 was orally administered 1/10 of LD50 of ethanolic extract of C. procera leaves (9.56 mg/kg b. wt), dissolved in 1% CMC, for 4 weeks and 8 weeks. Group 6 was orally administered 1/20 of LD50 of abamectin (0.44 mg /kg b. wt) dissolved in 1% CMC for 4 weeks and 8 weeks. LD50 of abamectin is 8.7 mg/kg body weight [21, 29]. Group 7 was orally administered 1/10 of LD50 of abamectin (0.87 mg/kg b. wt) dissolved in 1% CMC for 4 weeks and 8 weeks.
2.5. Samples Preparation
- After 4 and 8 weeks, six animals of each group were sacrificed under diethyl ether anesthesia. Blood samples were obtained from cervical vein. Blood samples were left to coagulate at room temperature and then they were centrifuged at 3000 rpm for 30 minutes. The clear non-haemolysed supernatant sera were quickly removed and divided into 3 portions. The obtained samples were kept in deep freezer at -30°C till used. One kidney was excised quickly, homogenized by using in isotonic solution (0.9% NaCl) at 10% concentration (w/v). The resultant homogenates were centrifuged at 3000 rpm and the homogenate supernatants were aspirated and fractioned into three portions, and then kept in deep freezer at -30oC till used. The other kidney was immediately excised and fixed in 10% neutral buffered formalin pending histopathological processing.
2.6. Biochemical Investigations
2.6.1. Detection of Serum Parameters Related to Kidney Function
- Serum urea and creatinine concentrations were detected by using reagent kits purchased from Diamond Company (Egypt) according to the method of Kaplan [30]. Uric acid concentration in serum was detected using reagent kit purchased from Biosystems Company (Spain) according to the method Barham and Trinder [31].
2.6.2. TNF-α and IL-4 Assays
- Serum levels of pro-inflammatory cytokine, TNF-α, and anti-inflammatory cytokine, IL-4, were determined by the quantitative enzyme immunoassay (EIA) kit purchased from R&D Systems (USA) according to the methods of Howard and Harada [32] and Croft et al. [33] respectively.
2.6.3. Assay of Oxidative Stress and Antioxidant Defense Markers
- Kidney glutathione and lipid peroxidation levels were determined by using reagents prepared in laboratory according to the methods of Beutler et al. [34] and Preuss et al. [35] respectively. Glutathione peroxidase (GPx), glutathione–S-transferase (GST) and superoxide dismutase (SOD) activities in kidney was assayed by using reagents prepared in laboratory according to the methods of Matkovics et al. [36], Mannervik and Guthenberg [37] and Marklund and Marklund [38] respectively.
2.7. Histological Investigation
- The kidney samples were excised, flushed with saline and then fixed in 10% buffered formalin. The fixed kidneys were transferred to Pathology Department, National Cancer Institute, Cairo University, Egypt for processing and staining. In brief, the fixed specimens were dehydrated, cleared in xylene and embedded in paraffin wax. Blocks were made, and 4 μm-thick sections were cut using a sledge microtome. The kidney tissue sections were deparaffinized, rehydrated and stained with hematoxylin and eosin (H&E). The stained slides were examined using bright field light microscopy to investigate the histoarchitecture of kidney.
2.8. Statistical Analysis
- The data obtained from the experiment were analyzed using one-way analysis of variance (ANOVA) [39] followed by LSD test to compare various groups with each other. Results were expressed as mean ± standard error (SE) and values of P>0.05 were considered non-significantly different while those of P<0.05 and P<0.01 were considered significant and highly significant respectively. F- Probability expresses the general effect between groups. Multi-factor analysis of variance (MANOVA) was also performed to evaluate the effect of time, dose and time-dose interaction.
3. Results
3.1. LD50 of Calotropis procera Latex in Albino Mice
- As indicted in table 1, the calculated LD50 after 72 hr of gradual increasing single oral doses of the latex obtained from C. procera was found to be 1.88 ml/ kg b. wt for albino mice. By using conversion table of Paget and Barnes [28], LD50 for rats was found to be 1.316 ml/kg b. wt. Based on this toxicity study, the orally administered dose in this study was chosen to be 66 µl/kg b. wt and 132 µl/kg b. wt that correspond to 1/20 of LD50 and 1/10 of LD50 respectively.
|
3.2. Effect on Serum Parameters Related to Kidney Function
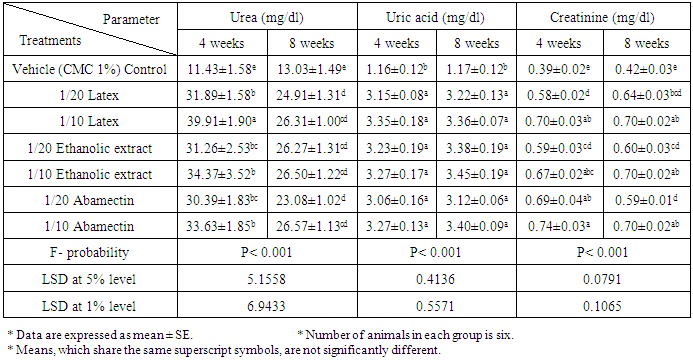
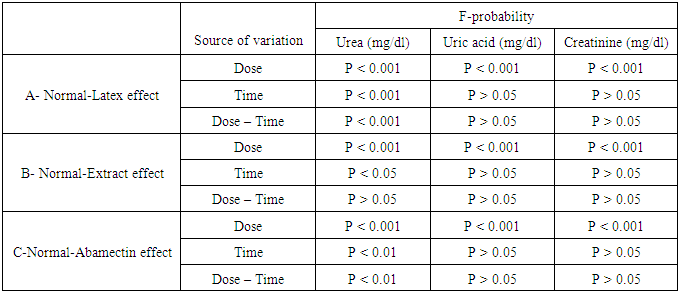
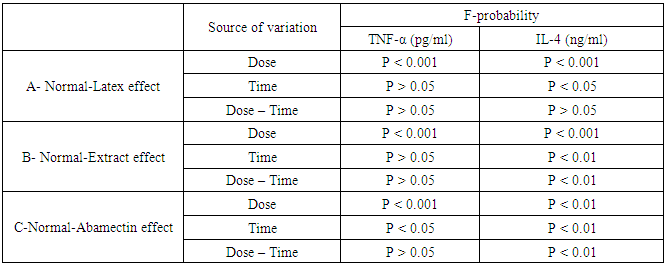
- As represented in table 2, serum urea, uric acid and creatinine concentrations were highly significantly (P<0.01; LSD) elevated after oral administration of latex, ethanolic extract and ABM for 4th and 8th weeks. One way ANOVA (Table 2) indicated that the general effect, between groups, on serum urea, uric and creatinine concentrations was very highly significant (P<0.001; F-probability). Two-way ANOVA (Table 3) depicted that the dose effect of C. procera latex, C. procera ethanolic extract and ABM, was very highly significant (P<0.001; F-probability) on serum urea, uric acid and creatinine levels. Time and dose-time interaction had insignificant effect (P>0.05; F-probability) on serum uric acid and creatinine levels as a result of the three tested materials. With regard to urea level, time and dose-time interaction had very highly significant effect (P<0.001; F-probability) with latex while they had significant effect (P<0.05; F-probability) and insignificant effect (P>0.05; F-probability) respectively with extract. The effect of time and dose-time interaction was highly significant (P<0.001; F-probability) with regard to normal-ABM effect (Table 3).
|
|
3.3. Effect on Serum TNF-α and IL-4 Levels
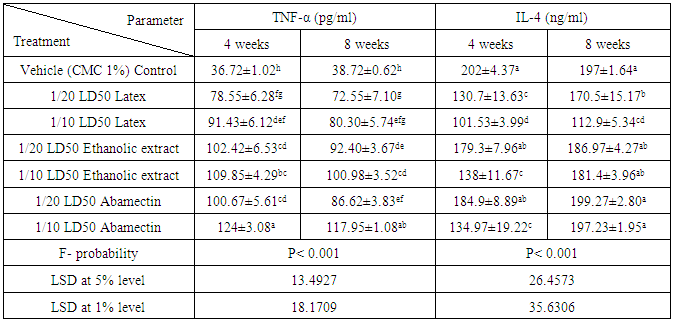
- Data represented in tables 4 and 5 depicted that all treatments induced strong adverse effects on the normal levels of serum TNF-α and IL-4 of normal rats. Administrations of latex, ethanolic extract of C. procera and ABM for 4 and 8 weeks induced a highly significant increases (P<0.01; LSD) in serum levels of TNF-α. On the other hand, while the higher doses of the ethanolic extract and ABM induced a highly significant effect on serum level of IL-4 after 4 weeks, the latex produced a significant effect as a result of the two tested doses after 4 and 8 weeks. ABM seemed to be the most effective in increasing serum TNF-α while latex is the most potent in lowering serum IL-4 level. One way ANOVA (Table 4) indicated that the general effect on serum TNF-α and IL-4 levels between groups was very highly significantly (P<0.001; F-probability) throughout the experiment. Two-way ANOVA (Table 5) revealed that the dose effect of latex and extract was very highly significant (P<0.001; F-probability). The dose effect of ABM was highly significant (P<0.01; F-probability) on IL-4 level while it was very highly significant (P<0.001; F-probability) on TNF-α level. The time and dose-time interaction of extract and ABM had a highly significant effect (P<0.01; F-probability) effect on IL-4 level. However, while time of administration of latex had significant effect (P<0.05; F-probability) on IL-4 level, its interaction with dose had insignificant effect (P>0.05; F-probability). The effect of time of ABM on TNF-α level was significant (P<0.05; F-probability) while its interaction with dose was insignificant (P>0.05; F-probability).
|
|
3.4. Effect on Kidney Oxidative Stress and Antioxidant Markers
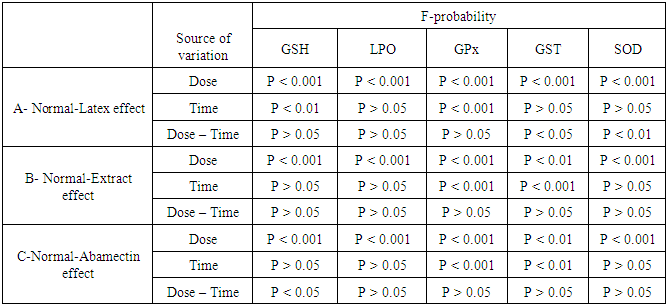
- The effects of latex, ethanolic extract of leaves of C. procera and ABM on kidney GSH level, LPO and GPx, GST and SOD activities are represented in tables 6 and 7. Kidney GSH level was significantly decreased after administration of the latex, extract and ABM at the 4th and 8th weeks; latex seemed to be more effective than extract and ABM. LPO was significantly elevated as result of high dose of latex and low and high doses of extract and ABM at the 4th week. As the period extended to 8 weeks, the effect on LPO was more progressed except for the group treated with low dose of ABM. Latex seemed to be the most effective in elevating LPO. With regard to the antioxidant enzyme, GPx activity in kidney was highly significantly decreased (P<0.01; LSD) after 4 and 8 weeks of administrations. The effect of the tested materials was more or less similar at 4th and 8th weeks of the experiment. The treatment with the plant and ABM induced a significant decrease in kidney GST activity at the 4th week. At 8th week, the low and high doses of latex as well as the high dose of ABM induced a significant decrease (P<0.01; LSD) in GST activity; the effect appeared to be dose-dependent and the latex seemed to the most effective. High dose of all tested materials induced a highly significant decrease (P<0.01; LSD) in kidney SOD activity after 4 and 8 weeks. The low doses also produced a highly significant decrease of SOD activity except for the low dose of ethanolic extract which induced insignificant effect. Latex in low dose at the 4th week appeared to be the most effective. One way ANOVA (table 6) indicated that the general effect on kidney levels of GSH and LPO and the activities of GPx, GST and SOD between groups was very highly significant (P<0.001; F-probability) throughout the experiment. Two-way ANOVA showed that the dose of latex, extract and ABM had a very highly significant effect (P<0.001; F-probability) on kidney GSH level, LPO and GPx and SOD activities while the dose effect of extract and ABM on kidney GST activity was highly significant (P<0.01; F-probability). Regarding latex administration, it was found that the time had an insignificant effect (P>0.05; F-probability) on LPO and GST and SOD activities, a highly significant effect (P<0.01; F-probability) on GSH level and a very highly significant effect (P<0.001; F-probability) on GPx activity. The interaction between dose and time had insignificant effect (P>0.05; F-probability) on kidney GSH level, LPO and GPx activity and highly significant effect (P<0.01; F-probability) on SOD activity. Concerning extract administration, it was depicted that time and dose-time interaction had insignificant effect (P>0.05; F-probability) on GSH level, LPO and SOD activity. However, while time had very highly significant effect (P<0.001; F-probability) on GPx and GST activities, the interaction between dose and time had insignificant effect (P>0.05; F-probability). With regard to ABM, time had insignificant effect (P>0.05, F-probability) on GSH level, LPO and SOD activity, a highly significant effect (P<0.01, F-probability) on GST activity and a very highly significant effect (P<0.001, F-probability) on GPx activity. Dose-time interaction had insignificant effect (P>0.05, F-probability) on kidney LPO and GPx, GST and SOD activities and significant effect on GSH level (P<0.05; F-probability) (Table 7).
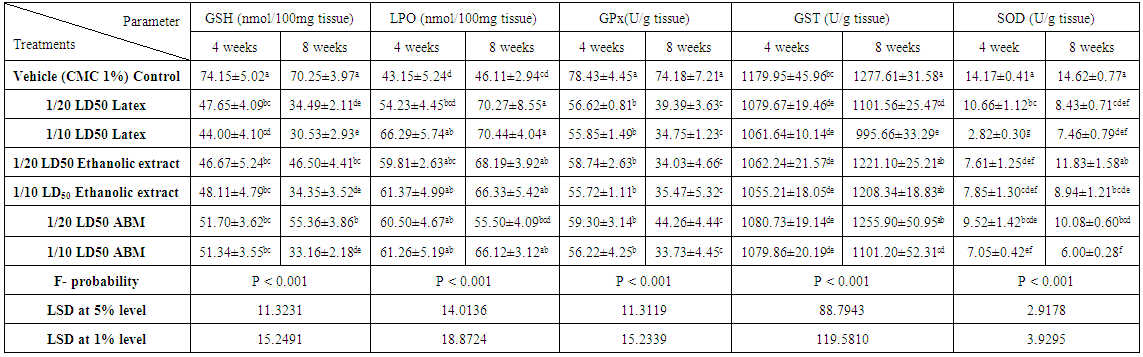 | Table 6. Effect of latex and ethanolic extract of C. procera and abamectin on kidney GSH, LPO concentrations, GPx, GST and SOD activities of normal rats |
|
3.5. Histopathological Effects
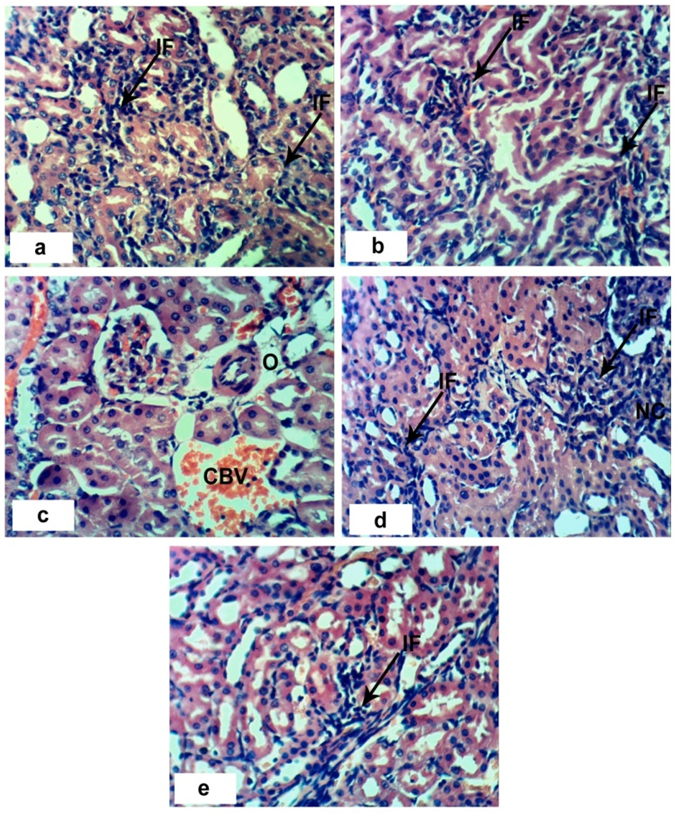
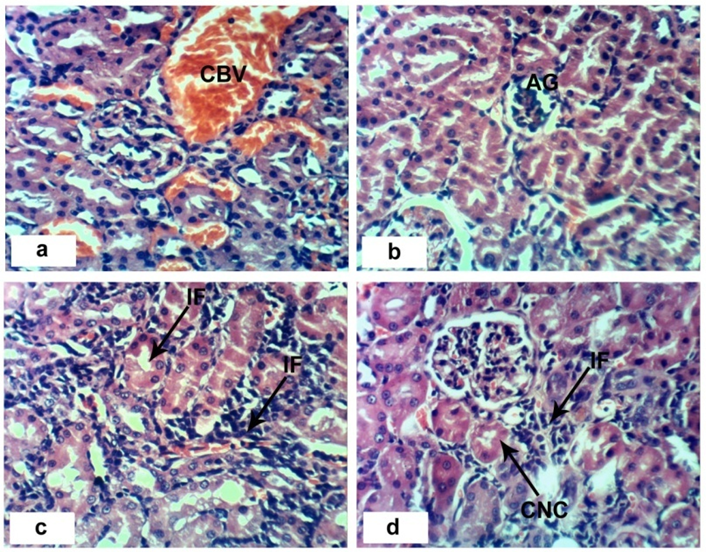
- Sections of the control rats′ kindeys demonstrated the normal histoarchitecture of the glomerulus and surrounding tubules (Proximal and distal tubules) (Figures 1a & b). As depicted in photomicrographs of kidney sections of rats at the 4th week (Figure 1a) and 8th week (Figure 1b), the kidney is divided into an outer layer, cortex and an inner layer, the medulla. In the cortex, there are glomeruli and proximal and distal convoluted tubules. The medulla contains, straight segments of distal tubules, collecting tubules and loops of Henle. Treatment of rats with 1/20 of LD50 of C. procera latex for 4 weeks caused vacuolation of endothelial cells lining glomerular tuft and perivascular edema associated with inflammatory cells infiltration (Figures 1c & d). Increasing period of administration to 8 weeks caused atrophy of glomerular tuft, perivascular odema associated with inflammatory cell infiltration and congestion of renal blood vessels (Figure 1e & f). Elevation of the dose to 1/10 of LD50 of latex interrupted the normal structure of the kidney; at 4th week, interstitial nephritis occurred (Figures 2a & b). Prolongation of the administration time of this dose (1/10 LD50) caused vacuolation and congestion of capillary tuft, focal necrosis associated with focal mononuclear cells infiltration as well as apoptosis of renal tubular epithelium (Figures 2c, d, e & f).Administration of 1/20 of LD50 of ethanolic extract of C. procera for 4 weeks caused absence of space in Bowman’s capsule (Figure 3a). After 8 weeks, this dose caused congestion of blood vessels, congestion of capillary tuft and perivascular odema associated with inflammatory cells infiltration (Figure 3b & c). The administration of the higher dose of ethanolic extract, 1/10 of LD50, for 4 weeks caused interstitial nephritis (IF) (Figure 3d). After 8 weeks of this dose administration, it induced focal necrosis associated with inflammatory cells infiltration, thickening of Bowman's capsule and edema (Figures 3e & f). Appearance of interstitial nephritis (Figure 4a) occurred after treatment with 1/20 of LD50 of ABM for 4 weeks. After 8 weeks of this dose administration, interstitial nephritis, edema and congestion of blood vessels (Figures 4b & c) were observed. 1/10 of LD50 of ABM administration for 4 weeks induced necrosis and interstitial nephritis (Figures 4d & e). Prolongation of period of 1/10 LD50 ABM administration to 8 weeks caused atrophy of glomerular tuft, interstitial nephritis and congestion of renal blood vessels and coagulated necrosis (Figures 5a, b, c & d).
4. Discussion
- C. procera plant commonly called Sodom apple or Giant milkweed belong to the family of Asclepidaceae. It is a major grazing plant found in Asian temperate region, Asia-tropical and Africa [40]. It was reported that ingestion of fresh C. procera leaves and latex has been suggested as toxic to many ruminants by several farmers [16, 18]. It was reported by Thankamma [41] and Basak et al. [42] that C. procera latex administered to rats revealed toxic, wound healing, and pain-killing effects. Chemical compounds in the latex are calotropagenin glycosides/derivatives, cardenolides, flavonoids, and saponins [43]. Cardenolides in the C. procera latex are associated with the toxic effects in mammals [44]. Phytochemical screening of the extracts of C. procera leaves indicated the presence of alkaloids, carbohydrates, cardiac glycosides, saponins, phenols, tannins, terpenoids and flavanoids which are known to possess medicinal and pesticidal properties [45]. It was reported by De Lima et al. [46] that the plant as hepatotoxic and cardiotoxic. Other researches have documented the renal toxicity in addition to hepatic toxicity of the plant [42, 47]. The chemical poisons from plants such as Argel (Solenostemma argel) and Usher (C. procera) are mostly alkaloids. Alkaloids are plant products, which are nitrogenous in nature. They are heterocyclic compounds having strong effects on the nervous system of animals and which may result in death [48].Oral administration of C. procera latex and C. procera ethanolic extract of leaves results in alterations of the kidney function markers. Serum creatinine, urea and uric acid levels increased significantly in rats treated with low and high doses of C. procera latex and ethanolic extract. These results are in line with those of Pouokam et al. [49] and Ahmad et al. [50] who found a significant increase of creatinine level in rabbits treated with C. procera. These results could be explained in the view of Pouokam et al. [49] who stated that creatinine is eliminated from the plasma through glomerular filtration and excreted as a waste product into urine. Elevation in creatinine concentration indicated the impaired renal function; possibly plant extracts ingredients may be accumulated into the kidney and may cause injuries to tubular epithelial cells and led to damage in kidney. Saponins disrupt cellular membranes [51] and cardenolides are specific inhibitors of the Na+/ K+ - ATPase. Presence of alkaloids, cardiac glycosides and tannins in various plants induces adverse effects on livestock [52].The negative impact of C. procera latex on the structural and functional integrity of kidney tissues was evidenced by the histopathological findings highlighting the damage after administration of latex. At the 4th week administration, latex induced vacuolization of the endothelial cells and perivascular edema associated with inflammatory cells infiltration. After increasing period of administration, latex caused atrophy of glomerular tuft, focal necrosis, mononuclear cells infiltration, apoptosis as well as congestion of blood vessels. The elevations of serum variables related to kidney function after extract administration were concomitant with the histological changes of kidney in the present study. Early histopathological changes at 4th week of extract administration include interstitial nephritis. As the period of administration extended to 8 weeks, the deleterious histopathological alterations were more pronounced and include congestion of blood vessels and capillary tuft, edema, inflammatory cells infiltrations as well as thickening of Bowman's capsule. These histological findings indicate that kidney damage caused by latex and extract is time dependent. The results are in contrast to several studies [18, 53] that reported hepatoprotective and renoprotective effect of the plant. Such discrepancies may be due to the difference in used doses, plant parts, extract preparation methods and animal species.The present results indicated that administration of 1/20 and 1/10 LD50 of abamectin significantly increased urea, uric acid and creatinine levels compared with control groups. Similar findings were demonstrated by Eissa and Zidan [54], Abd-Elhady and Abou-Elghar [55]. Uric acid and creatinine levels are useful in early deduction of nephrotoxicity induced by exogenous compounds. The current results are explained as in the view of Abd-Elhady and Abou-Elghar [55] who reported that the oral administration of 1/10 and 1/30 LD50 of abamectin for 30 and 210 days respectively induced marked increase in urea and creatinine concentrations compared with controls. The treated animals′ elevation of uric acid and creatinine concentrations may be attributed to the reduction in glomerular filtration in the kidney. Such elevation also reflects the dysfunction of the kidney tubules.The increase of uric acid concentration is an indicator of impaired kidney function since the organ primarily excretes urea in the urine. The increase in creatinine level due to abamectin administration was also correlated closely with the histopathological changes in the kidney. Early histopathological changes at 4th week of ABM administration include interstitial nephritis. As the period of administration extended to 8 weeks, the deleterious histopathological alterations were more deteriorated and they include congestion of blood vessels and capillary tuft, edema, inflammatory cells infiltrations congestion and atrophy of capillary tuft. These changes provide evidence that kidney damage caused by ABM is time dependent.The milky sap is a mixture of various chemicals including calotropis glycosides such as calotropin, calotoxin, calactin, uscharidin, voruscharin which are caustic in nature and are considered poisonous. The irritant and pro-inflammatory property of latex of C. procera has been well established [56]. Accidental exposure to the latex has been reported to cause inflammation of the skin and eyes [57, 58].In the present study, both the latex and ethanolic extract induced marked inflammations in rats which were observed in histopathological lesions in kidney and were evidenced by the increased level of serum pro-inflammatory cytokine, TNF-α and depletion in the level of serum anti-inflammatory cytokine, IL-4. Increasing the dose led to increase in the adverse effects on TNF-α, and IL-4. Administration of low dose of ABM markedly increased TNF-α and decreased IL-4 level in the present study. The depletion in IL-4 level was more progressed at the higher dose (1/10 of LD50).Reactive oxygen species (ROS) are produced by living organisms as a result of normal cellular metabolism. At low to moderate concentrations, they function in physiological cell processes, but at high concentrations, they produce adverse modifications to cell components, such as lipids, proteins, and DNA [59]. The shift in balance between oxidant/ antioxidant in favor of oxidants is termed oxidative stress. Oxidative stress contributes to many pathological conditions. When oxidative stress occurs, cells attempt to counteract the oxidant effects and restore the redox balance by activation or silencing of genes encoding defensive enzymes, transcription factors, and structural proteins [60]. Glutathione (GSH) is highly abundant in all cell compartments and is the major soluble antioxidant. It detoxifies hydrogen peroxide and lipid peroxides via action of glutathione peroxidase. GSH donates its electron to H2O2 to reduce it into H2O and O2. GSH protects cells against apoptosis by interacting with proapoptotic and antiapoptotic signaling pathways [61]. Lipid peroxidation is the oxidative deterioration of polyunsaturated lipids to form radical intermediates that bring about cellular damage. Malondialdehyde (MDA), a major end product of this reaction, is an index of lipid peroxidation and has been estimated as thiobarbituric acid reactive substances (TBARS) [62].In this study, it is observed that administration of latex and ethanolic extract as well as ABM induced marked decrease in GSH level and kidney GPx, GST and SOD activities and on the other hand, they induced significant increase in lipid peroxidation. These results are in line with those of El-Shenawy [63] who studied the toxic effect of ABM on isolated rat hepatocytes and found that ABM decreased GSH concentration and GPx and SOD activities. The suppression in antioxidant defense system and activation of lipid peroxidation may play a crucial role in cellular damage in many organs including the kidney. This suggestion is supported by the present findings which revealed that the deteriorations in the antioxidant defense system and augmented oxidative stress are associated with the elevated levels of serum markers of kidney damage and dysfunction. As indicated in the present study, C. procera latex seemed to have more deleterious effects on the oxidative stress and antioxidant defense system than C. procera latex leaves extract and ABM. Based on the findings of the present study, it can be concluded that the nephrotoxicity of C. procera leaves and latex as well as ABM is due to the elevation in lipid peroxidation, depletion in antioxidant levels and stimulation of the inflammatory status. Thus, this study calls for collaboration between pharmacognosists and biologists for further investigations to assess the toxic effects of various constituents of latex and leaves ethanolic extract.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML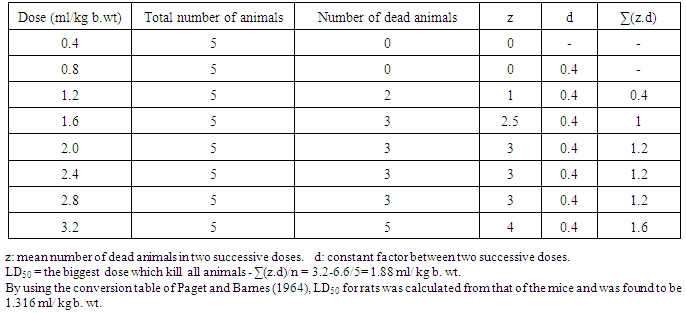
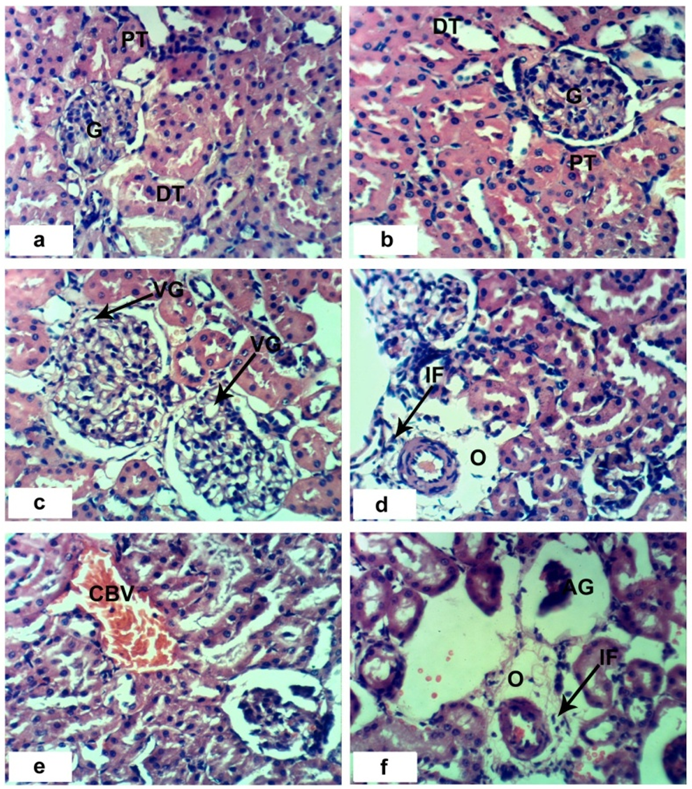
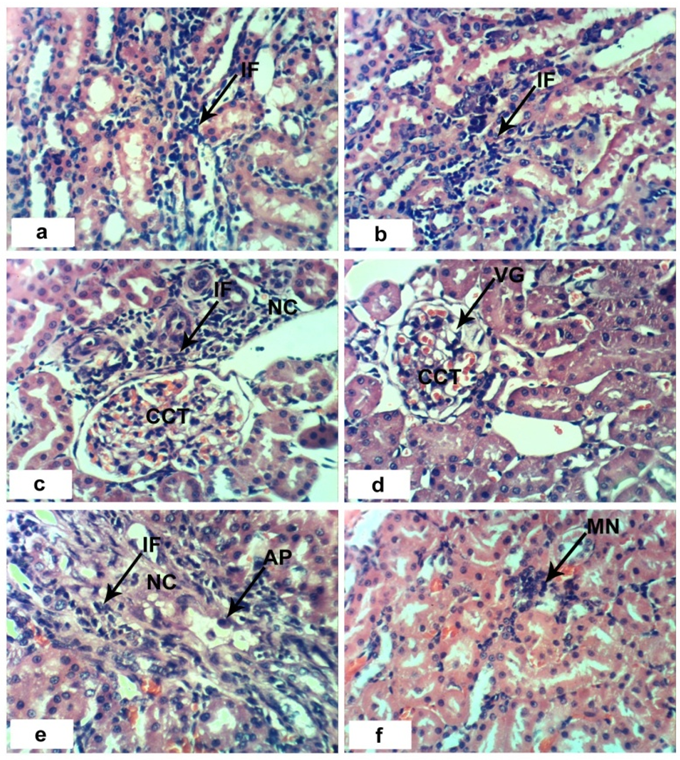
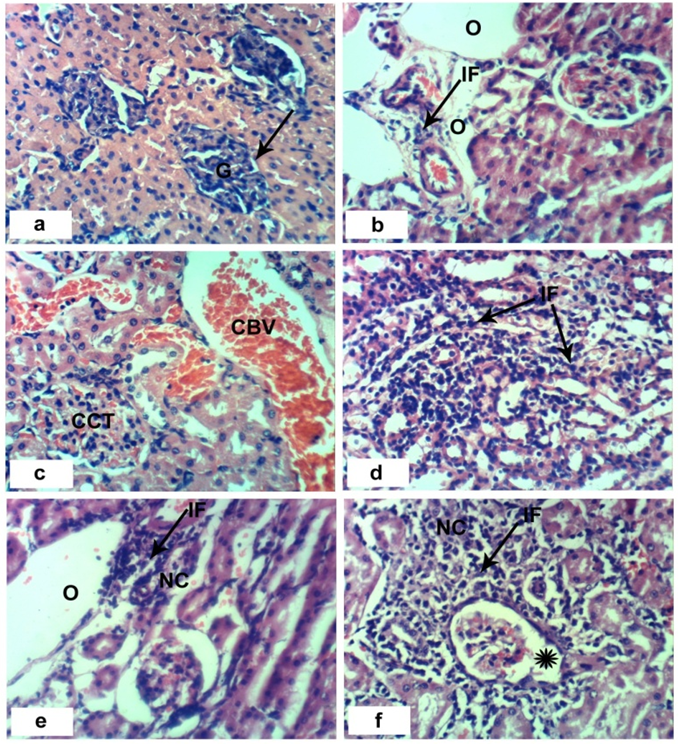
 ). b and c: sections of rats treated with 1/20 LD50 extract for 8 weeks showing perivascular odema (O) associated with inflammatory cells infiltration (IF), congestion of capillary tuft (CCT) and congestion of renal blood vessels (CBV). e and f: sections of rats treated with 1/10 LD50 extract for 8 weeks showing focal necrosis (NC) associated with inflammatory cell infiltration (IF), odema (O) and thickening of Bowman’s capsule (*). (x 400)
). b and c: sections of rats treated with 1/20 LD50 extract for 8 weeks showing perivascular odema (O) associated with inflammatory cells infiltration (IF), congestion of capillary tuft (CCT) and congestion of renal blood vessels (CBV). e and f: sections of rats treated with 1/10 LD50 extract for 8 weeks showing focal necrosis (NC) associated with inflammatory cell infiltration (IF), odema (O) and thickening of Bowman’s capsule (*). (x 400)