-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2016; 6(3): 57-65
doi:10.5923/j.ajmms.20160603.01

Plasma Apelin Concentrations in Non-obese Acute Myocardial Infarction Patients with Type 2 Diabetes Mellitus
Ashraf T. Abd Elmouttaleb1, Eman E. Ebrahem2, Kamal A. Marghany3, Ebrahim M. Bayomy4, Abdelhaleem A. Hassabo5
1Medical Biochemistry Department, Assisted Reproductive Unit. International Islamic Center for Population Studies and Research, Al-Azhar University, Egypt
2Medical Biochemistry Department, Faculty of Medicine (for girls), Al-Azhar University, Egypt
3Cardiology Department, Faculty of Medicine, Al-Azhar University, Egypt
4Clinical Pathology Department, Faculty of Medicine, Al-Azhar University, Egypt
5Internal Medicine Department, Faculty of Medicine, Al-Azhar University, Egypt
Correspondence to: Ashraf T. Abd Elmouttaleb, Medical Biochemistry Department, Assisted Reproductive Unit. International Islamic Center for Population Studies and Research, Al-Azhar University, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: The discovery of the apelin - APJ axis is an exciting development in cardiovascular research. Apelin is thought to play roles in cardiovascular functions and volume regulation like vasodilation and decreased blood pressure; vasoconstriction in the presence of dysfunctional endothelium; positive inotropic effects; inhibition of antidiuretic hormone (ADH) release; dilation of afferent and efferent arterioles, and vasoconstrictive effects on smooth muscle cells. Objective: This study was designed to identify the association between apelin concentrations and acute phase of myocardial infarction in patients with type 2 diabetes mellitus and without. Subjects and Methods: This study included (90) individuals, (60) patients with acute myocardial infarction (AMI) (30) patients had type 2 diabetes and (30) patients of them had normal blood glucose, (30) individuals with atypical chest pain normal ECG and normal coronary angiography were included as a control group. Plasma apelin was measured using Apelin microplate ELISA assay kit. Results: The mean plasma apelin in AMI diabetic and non-diabetic patients were (1.21±0.72, 1.24±0.76) respectively which were significantly lower compared to control group (2.67± 0.8), p < 0.001. There is no significant difference between diabetic and non-diabetic groups, p=0.5. There were positive significant correlations between plasma apelin and BMI and HDL-cholesterol in both AMI groups. There were negative significant correlations between plasma apelin and LDL-cholesterol, triglyceride and CRP in both AMI groups. Conclusions: The present study showed that decreased plasma apelin level was associated with AMI in non obese patients with or without type 2 diabetes mellitus and it was not related to age, BMI, LDL-cholesterol, TG, HbA1c, CRP and other coronary artery disease risk factors.
Keywords: Apelin, AMI, Diabetes mellitus, Coronary artery disease
Cite this paper: Ashraf T. Abd Elmouttaleb, Eman E. Ebrahem, Kamal A. Marghany, Ebrahim M. Bayomy, Abdelhaleem A. Hassabo, Plasma Apelin Concentrations in Non-obese Acute Myocardial Infarction Patients with Type 2 Diabetes Mellitus, American Journal of Medicine and Medical Sciences, Vol. 6 No. 3, 2016, pp. 57-65. doi: 10.5923/j.ajmms.20160603.01.
Article Outline
1. Introduction
- Apelin is produced and secreted by adipocytes in humans indicating that it is an adipokine, also by vascular smooth muscle, pancreas and pituitary gland [1].Apelin is widely expressed in various tissues, including gastrointestinal tract, adipose tissue, brain, liver, heart, lungs, kidneys and cardiovascular system, which occurs in vascular smooth muscle, endothelial cells and cardiomyocytes. Apelin production in adipose tissue is regulated by factors, such as fasting and refeeding, insulin, hypoxia, growth hormone and TNFα [2].Apelin is a peptide isolated from bovine stomach extracts acts as the endogenous ligand for the orphan G-protein coupled receptor, named APJ, derived from 77 amino acid long preproprotein that is cleaved intracellularly to produce a number of secreted bioactive peptides, all of which retain the C-terminus of the preproprotein ,which represents the biological activity of apelin. It produces five active isoforms (apelin 12, 13, 17, 19 and 36), each showing different receptor binding affinities; the gene has been localized on the X chromosome Xq25-q26.3 [3].The apelin receptor APJ is one of a group of G-protein coupled receptors (GPCR) that have recently been paired with their cognate peptide ligands [4].Genes related to human APJ have been identified in the rat, mouse, monkey, fish, and frog. In human, the gene maps to band q12.1 of chromosome 11 [5].Apelin 13 and apelin 36 differ in receptor binding affinity. Apelin-13 which corresponds to the sequence 65-77, was found to be the predominant isoform in human cardiac tissue due to higher resistance to degradation [6].Apelin/APJ system exerts a broad range of physiological and pathological actions, including angiogenesis, vasodilatation and vasoconstriction, myocardial ischemia, hypertension, appetite, fluid homeostasis, retinal vascular development and neovascularization, neuroprotection, cell proliferation, and glucose utilization. Therefore it is capable of interfering with diabetes and obesity [7].Whereas insulin stimulates adipose tissue apelin expression, apelin inhibits insulin secretion, presenting an interesting interaction between the two systems. Interestingly, apelin-13 has been found to have beneficial effects on high fat diet induced obese mice, improving glucose tolerance and increasing glucose utilization in normal and insulin- resistant mice [8].Considering the physiological actions of apelin in the control of glucose homeostasis, it is tempting to propose a link between apelin and obesity associated insulin resistance. Accordingly, apelin overproduction in the obese might represent a protective mechanism before the emergence of type2 diabetes or cardiovascular diseases. Thus, apelin becomes a potential therapeutic target in diabetes and obesity [9].In pancreas, apelin inhibits the insulin secretion induced by glucose. This inhibition reveals the functional interdependency between apelin signaling and insulin signaling observed at the adipocyte level where insulin stimulates apelin production [10].Apelin signaling mediates important effects in cardiovascular homeostasis, this was suggested by observing distribution of mRNA encoding both receptor and ligand in human and rat tissues. They were expressed in peripheral rat and human tissues, including small resistance vessels and large conduit arteries and veins. Apelin is a vasodilator employing human arteries, veins and resistance vessels. Accordingly, intravenous administration in rodents reduces mean arterial pressure, systemic venous tone and cardiac preload and afterload. Such vasodilation is endothelium dependent and predominantly mediated through nitric oxide (NO) dependent pathways [11].Apelin increased the activity of sarcolemmal Na+/H+ exchanger, leading to a positive inotropic effect without inducing myocardial hypertrophy [12].In heart failure, apelin prevents the progression of cardiac hypertrophic remodeling induced by pressure overload by inhibiting reactive oxygen species production, and stimulating catalase activity; these results suggest that apelin is a potent regulator of cardiomyocyte antioxidant reserve against oxidative stress [13].Apelin may be a candidate for the treatment of atherosclerosis. Considering the role of promoting monocytes adhesion, vascular smooth muscle proliferation and angiogenesis, apelin/APJ may be more effective as a therapeutic target. So, more experiments directly in atherosclerosis model instead of atherosclerotic dangerous situations or hallmarks are essential to reveal the substantial role as well as the pathogenesis of apelin in atherosclerosis [14].At present, apelin represents an angiogenic factor similar to vascular endothelial growth factor (VEGF) and has been investigated as a new target to limit tumor angiogenesis. Indeed, apelin is overexpressed in one third of human tumors and promotes lymphangiogenesis and tumor growth in vivo. Thus, its inhibition might represent an interesting mechanism to restrain tumor growth [15].Conversely, apelin's angiogenic property, which affect both existing and newly developing blood vessels, together with its capacity to enhance superoxide dismutase activity and to protect against ischemic heart disease after hypoxia-reperfusion might be helpful for functional recovery after ischemia [16]. This study was designed to identify the association between apelin concentrations and acute phase of myocardial infarction in patients with type 2 diabetes mellitus and without.
2. Subjects and Methods
- This study was conducted at Bab-El Sharia University Hospital from May (2015) to November (2015). It included (90) individuals, (60) patients with acute myocardial infarction (AMI) (30) patients had type 2 diabetes and (30) patients of them had normal blood glucose, (30) individuals with atypical chest pain, normal ECG and normal coronary angiography were included as a control group. Age of patients varied between 50-64 years old. The patients selected after approved diagnosis of AMI and rule out of other patients with exclusion criteria. AMI was defined as resting chest pain lasting more than 30 minutes accompanied by ischemic electrocardiographic changes and was confirmed by the presence of total creatine kinase or creatine kinase isoenzyme (CK-MB) fraction levels of more than three times the upper limit of normal or cardiac troponin T greater than the upper limit of normal [17]. A written informed consent was taken from each studied patient after full explanation of the purpose of the study.Exclusion criteria: Obesity, dyslipidemia, renal failure, type I diabetes, valvular heart disease, conductive disturbance, thyroid dysfunction, history of infectious disease, collagen vascular disease such as systemic lupus erythematous, scleroderma, rheumatoid arthritis, liver cirrhosis, hepatitis, malignancy, history of organ transplantation, bleeding disorders, HIV or prior chest radiation. Height and weight were measured and body mass index [weight (kg) / height (m)²] was used as estimate of overall and central obesity, patient with BMI >30 were excluded and BMI less than 25 defined as normal, 26-30 as overweight and above 30 as obese.- For diabetes patients who had fasting blood glucose of >126 mg/dl or received DM treatment were diagnosed as having DM. - Blood pressure was measured two times in sitting position after 5 minute of rest using sphygmomanometer and hypertension was defined as blood pressure more than 140/90 mmHg or the use of anti-hypertensive medication.- Blood samples were obtained by vein puncture and collected in liquid EDTA blood tubes within 24 hours from onset of acute myocardial infarction, whole blood was used for the analysis of glycated hemoglobin (HbA1c), then it was centrifuged and separated plasma were stored at-80°C till assay time. - Serum was obtained by collecting blood into plain vacutainer tubes.- Serum glucose levels were measured by glucose oxidase method.- Serum Total cholesterol and triglycerides were measured by enzymatic technique.- HDL- cholesterol level was measured after precipitation of the other lipoprotein with heparin- manganese chloride. - LDL- cholesterol level was estimated using the formula of Friedewald et al., 1972 [16]: (LDL-C) = [(total cholesterol) – (HDL-C) – (triglyceride/5)].- Glycated hemoglobin (HbA1c) was determined by cation exchange chromatography using BioSystem S.A Spain kit and was quantified by direct photometric reading at 415 nm.- Highly sensitive CRP (hs-CRP) test was performed by using the Boering BN-II (Dade Boering, Marburg, Germany) nephelometer. The lower limit of hs- CRP detection by the Dade Bowering nephelometer is 0.17 mg/L.- Troponin-T was measured by Cobas 411 Roche, GmbH, Germany.- Plasma apelin was measured using (Apelin-12) microplate ELISA assay kit (Glory Science co., Del Rio, USA), this APA12 ELISA kit includes a set of calibration standards. The calibration standards are assayed at the same time as the samples and allow operators to produce a standard curve of Optical Density versus AP12 concentration. The antibody used in this apelin assay cross-reacts 100% with apelin 12, 13 and 36. The assay therefore includes all of the above peptides if present in the plasma. The sensitivity of the assay was 0.1ng/ml.
3. Statistical Analysis
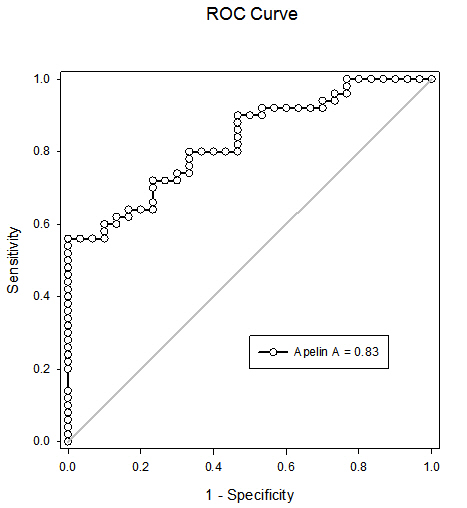
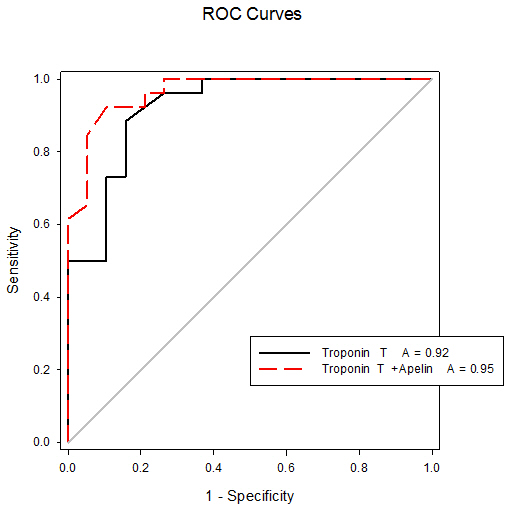
- All data were analyzed using a SPSS software package (version 17.0, SPSS Inc. Chicago, IL, USA). Continuous variables were expressed as mean ± SD. Comparison of apelin level was performed using ANOVA test. Pearson correlation coefficients were used to explore relationships between apelin level and different clinical and laboratory data. P value of < 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curve was used to detect the cut-off value for plasma apelin that predict patients with AMI.
4. Results
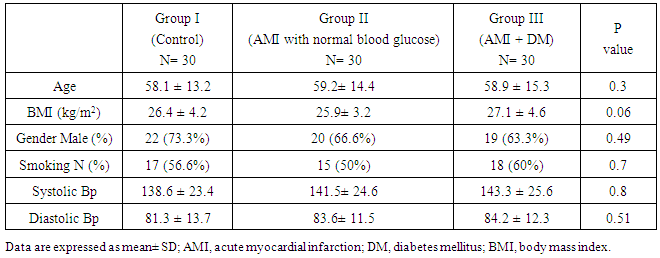
- In Table (1): There were non-significant differences between studied groups regarding demographic and clinical data.
|
|
|
5. Discussion
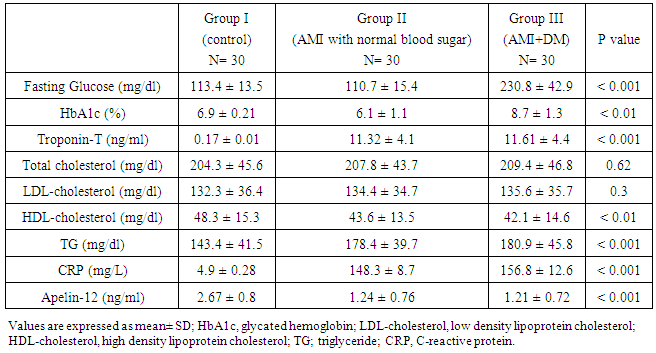
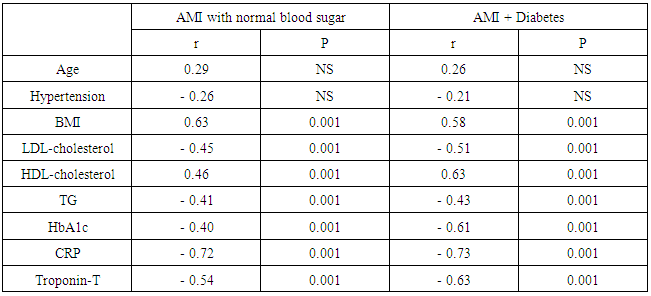
- Recently, it has become apparent that adipose tissue is an active endocrine and paracrine organ that releases several bioactive mediators [19]. The discovery of the apelin - APJ axis is an exciting development in cardiovascular research. Apelin is thought to play roles in cardiovascular functions and volume regulation like vasodilation and decreased blood pressure; vasoconstriction in the presence of dysfunctional endothelium; positive inotropic effects; inhibition of antidiuretic hormone (ADH) release; dilation of afferent and efferent arterioles, and vasoconstrictive effects on smooth muscle cells [20]. Increased apelin expression has been found in cardiovascular tissues, cardiomyocytes, vascular smooth muscle cells, and endothelial cells. Notably, apelin recently has been implicated in cardiovascular system physiology in regard to endothelium – dependent vasodilation, cardiac contractility, and the reduction of vascular wall inflammation [21]. Apelin peptides involves widely in the enzymatic reactions or cascades of renin angiotensin aldosterone system (RAAS) and it also acts as antagonist or counter regulatory towards the action of angiotensin-2 supports the possible role of apelin in vascular reactivity maintenance in diabetes, also cardiovascular diseases and blood pressure maintenance. Any dysfunction or abnormalities in apelin-apelin receptor (APJ) signaling pathway contribute the decreased vasodilation and increased vasoconstriction responses and also related to insulin resistance related disorders [22]. The wide distribution of apelin and APJ throughout the body and its predominant localization in vascular endothelium mirror the distribution of angiotensin-II receptor type I (AT1).The physiological effects of apelin (positive inotropism, vasodilation, decreased blood pressure and diuresis through effect on central nervous system) are opposite to the actions of angiotensin-II. Therefore apelin acting through APJ receptor may modulate the detrimental effects of AT1 activation and help to prevent progressive left ventricular systolic dysfunction and the onset of heart failure [23].Apelin also cross the blood brain barrier by designing a small molecule non peptide mimetic of apelin action for penetration into blood brain barrier. Central apelin actions on ADH (decrease ADH release) levels contribute the positive effects of apelin receptor agonist on blood pressure by acting on the respective neuron on hypothalamus [24].The apelin plays an emerging role in medicine and biology and mainly involved in the regulation of cardiovascular function and fluid homeostasis. Accumulating evidence supports apelin involvement in cardiovascular function, but its causative relationship with ischemic heart disease is controversial [25].Limited evidence has emerged, indicating the association of reduced apelin with coronary atherosclerosis, so in our study apelin as a novel biomarker of coronary atherosclerosis development and severity was studied to prove its association with atherosclerosis and acute myocardial infarction.In the present study the mean plasma apelin in AMI diabetic and non-diabetic patients were (1.21±0.72, 1.24±0.76) respectively which were significantly lower compared to control group (2.67± 0.8), p < 0.001. There was no significant difference between diabetic and non-diabetic groups p=0.5.Our results are in agreement with results of Weir et al [26] who reported that decrease of apelin levels early after AMI, with a progressive elevation over time, they showed significant increase of apelin from base line to 24 week after AMI. Decreased levels of apelin -36 during 5 days interval following ST elevation myocardial infarction have been reported by Kuklinska et al [27].Kadoglou et al [28] showed that both groups of patients with unstable angina and acute myocardial infarction had significantly lower level of apelin compared to patients with asymptomatic coronary artery disease. Moreover, they confirmed the correlation of low apelin concentration with a coronary artery disease and severity angiographically quantified by the Gensini Score and the number of narrowed vessels, and concluded that the latter relationship was independent of other traditional cardiovascular risk factors.This finding was confirmed in another study conducted among subjects with stable angina by Li et al [29] who showed that the plasma aplin levels in patients with stable angina pectoris were lower than the controls.Moreover Malyszko et al [30] found apelin levels lower in hemodialysis patients with ischemic heart disease than those without ischemic heart disease.Abdelaziz et al [31] reported that plasma apelin levels were significantly decreased in coronary artery disease patients compared to controls and apelin levels increased after percutaneous coronary intervention which could be an adaptive reaction for the endothelium recovery.Földes et al [32] demonstrated that plasma apelin levels were significantly decreased in patients with heart failure due to coronary heart disease compared to normal subjects. In addition, Chong et al [33] reported that plasma apelin concentration is decreased in patients had chronic heart failure and about 50% of the investigated patients had chronic heart failure due to ischemic heart disease.On the other hand, our results are not in agreement with the results of Karadag et al [34] who reported that the levels of plasma apelin were lower in patients with ischemic heart disease, but the difference was not statistically significant.Also our results are not in agreement with the study of Abd-Elbaky et al [35] who stated that plasma apelin levels were elevated in type 2 diabetes mellitus patients with cardiovascular disease compared to controls and type 2 diabetes without cardiovascular disease (CVD) its elevation represented 2.5 and 1.5 folds controls and type 2 diabetes mellitus without (CVD) respectively.The exact mechanism of the cardioprotective effects of apelin on cardiovascular system cannot be established. Several possible roles of apelin should be considered. First: Apelin-13, the predominant circulating apelin isoform significantly promoted intracellular cholesterol efflux and reduces macrophage foam cell formation, indicating a potential anti atherogenic function Liu et al [36]. Moreover, Kadoglou et al [28] have demonstrated lower apelin levels in patients with carotid atherosclerosis as compared to healthy control, and that apelin increment is independently associated with atorvastatin related carotid plaque stabilization.Pitkin et al [37] have shown an increase in apelin expression in atherosclerotic coronary artery, with additional peptide localizing to the atherosclerotic plaque. Apelin receptors were also found to be present within atherosclerotic plaque and to have a similar distribution to its ligand. Increased content of apelin and its receptor might be an indicator of increased anti-inflammatory activation of macrophages thereby limiting plaque instability.Apelin treatment decreased aortic aneurysm formation in mice by decreasing macrophage infiltration, suggesting that apelin may be beneficial in human atherosclerosis by a similar mechanism as reported by Leeper et al [38].Second: Apelin may limit atherosclerosis progression by inhibiting angiotensin-II effects on the vasculature by blood pressure maintenance. Any dysfunction or abnormalities in apelin- apelin receptor (APJ) signaling pathway contribute to decrease vasodilation and increase vasoconstriction responses [22]. In contrast to angiotensin a potent vasopressor and anti-diuretic hormone (ADH), apelin both lowers the blood pressure and also stimulates diuresis by inhibition of arginine vasopressin activity and release [39].Third: Apelin is a direct coronary vasodilator that increases myocardial contractility in human via nitric oxide dependent mechanisms and causes a reduction in left ventricular end-diastolic pressure. Intravenous injection of apelin lower blood pressure by triggering the release of nitric oxide from endothelial cells [40]. Li et al [29] reported that apelin may have a regulatory effect on proliferation of vascular smooth muscle cell and nitric oxide production.Fourth: Injected apelin exerts acute anti-inflammatory effect on vascular system, injected apelin reduces the mRNA levels of pro inflammatory markers (MCP-1, macrophage inflammatory protein-1α, IL-6 and tumor necrosis factor-α), and the plasma levels of apelin are inversely correlated to inflammatory markers (C-reactive protein and IL-6) [41].Fifth: Hypoxia regulates apelin gene expression and secretion in cardiomyocytes. Hypoxia induction of apelin could be a part of acute response of the heart muscle to an impaired oxygen supply, such as at the time of myocardial infarction. Myocardial gene expressions as well as secretion of apelin are activated by hypoxia via activation of hypoxia-inducible factor-1(HIF-1), it has been shown that increased apelin expression could therefore serve as an adaptive mechanism to maintain the contractile function of the heart as well as over expression of (HIF-1) reduced infarct size and limited the progression of infarct-induced cardiac failure [42].Sixth: Apelin considerably protects the heart against ischemia-reperfusion (I/R) injury. Gao et al [43] have suggested that apelin or pharmacological agonist of APJ receptors could act as novel approach for attenuating myocardial ischemia and reperfusion injury in patients with coronary artery disease. Recent interesting experimental data have indicated that expression of apelin-APJ pathway during differentiation of bone marrow mononuclear cells (BMSCs) into cardiomyogenic cells may be an important mechanism in regulation of myocardial regeneration and its functional recovery after acute myocardial infarction [43]. Simpkin et al [44] for the first time demonstrated the protective effects of apelin against I/R injury in rodents through the perfusion injury salvage kinase (RISK) pathway activation. They reported that apelin administration has a cardioprotective effect against reperfusion injury, with reduction in the infarct size and reduced cardiomyocyte membrane damage. Treatment of human umbilical vein endothelial cell with apelin dose – dependently augments angiogenic responses [45].Kidoya et al [46] indicated that apelin together with vascular endothelial growth factor (VEGF) efficiently induced functional vessels larger than VEGF alone, in the hind limb ischemia model of mice. If the effect of apelin on cardiac collateralization is proved in future studies, it can be considered as a valuable factor for the patients with ischemic heart failure. A study of Pisarenko et al [47] demonstrated cardioprotective properties of exogenous apelin-12 analogues in isolated working rat heart subjected to global ischemia and reperfusion, treatment with apelin-12 increased recovery of cardiac function and coronary blood flow during reperfusion compared with control. They are manifested by enhanced contractile and pump function recovery and accompanied by better restoration of myocardial energy state (enhanced restoration of myocardial ATP, adenine nucleotide pool, phosphocreatine and reduced accumulation of myocardial lactate).The angiogenic activity of apelin may result from the combination of the molecule to APJ, playing a role in the proliferation, migration, and tube formation of endothelial cells. In addition, apelin activate cell transduction cascades such as serine/threonine kinase (AKT) and extra cellular signal- regulated kinases which lead to proliferation of endothelial cells and the formation of new blood vessels [48].In this study the mean plasma CRP level in AMI diabetic and non-diabetic patients were (156.8±10.6, 148.3±8.7) respectively which were significantly higher compared to control group (4.9±0.28). These results agree with the result of Gupta et al [49] who found association between serum CRP level and multiple measures of atherosclerosis in coronary heart disease. Arroyo et al [50] have demonstrated that C-reactive protein levels were associated with coronary events. The study of Kadoglou et al [28] has demonstrated that high sensitive CRP, LDL-C, and diabetes to be independent determinant of low apelin levels among patients with coronary artery disease. Also in the current study we found significantly negative correlation between plasma apelin and C-reactive protein levels in acute myocardial infarction groups, these results support a theory of previous studies demonstrated cardioprotective effect of plasma apelin as anti-inflammatory effects on vascular system. El-shehaby et al [51] found a negative correlation of apelin levels with interleukin-6 and hs-CRP levels. In addition, the study of Horiuchi et al [52] reported that apelin has anti- inflammatory, anti-infectious and inhibitory effects on inflammatory mediator's release.In this study plasma apelin levels were significantly negative correlated with LDL-cholesterol and triglyceride levels this agrees with the results of Tasci et al [53] who reported that in human with hypercholesterolemia, plasma apelin was decreased compared to matched controls, and lowering LDL-cholesterol with life style changes and /or statins resulted in an increase in apelin levels.In contrary to our study, the study of Abdelaziz et al [31] who found no significant correlations between plasma apelin levels with age, gender, hypertension, plasma cholesterol, BMI, diabetes mellitus and smoking.
6. Conclusions and Recommendations
- In the present study the plasma apelin levels were decreased in non-obese acute myocardial infarction patients (diabetic and non-diabetic compared to the control group. In addition no significant difference was found between diabetic and non-diabetic, suggesting that plasma apelin is an independent determination of coronary heart disease regardless to blood glucose level. Apelin might play an additional protective role against cardiovascular complications of diabetes mellitus. Plasma apelin was found to be independent from traditional cardiovascular risk factors age, BMI, LDL-cholesterol, triglyceride and CRP. In our study the combination of troponin-T and apelin has increased the sensitivity of troponin-T for predicting AMI to 95.7%; further studies including large number of patients are needed to evaluate predictive and diagnostic values of plasma apelin levels for clinical use as well as the pathogenic importance of this biomarker in plaque instability and rupture. Future studies for the use of apelin for therapeutic administration in ischemic heart disease are recommended.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML