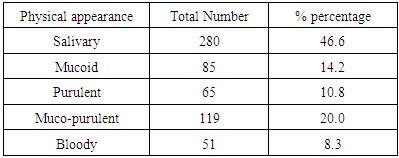-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(6): 287-291
doi:10.5923/j.ajmms.20150506.04

Prevalence of Pulmonary Tuberculosis (PTB) in Minna and Suleja Niger State, Nigeria
Sani R. A. , Garba S. A. , Oyeleke S. B. , Abalaka M. E.
Federal University of Technology, Minna, Nigeria
Correspondence to: Sani R. A. , Federal University of Technology, Minna, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study was aimed at determining the prevalence of tuberculosis in Minna and Suleja area of Niger State. The Prevalence of pulmonary tuberculosis in Minna and Suleja areas of Niger State was studied using standard microscopic technique and sputa culturing procedure. Ziehl Neelsen’s staining technique was used to determine the presence of acid fast bacilli (bacteriologically confirmed cases of sputum smear-positive tuberculosis). The isolation of acid-fast bacilli (AFB) from sputa of Patients that comes with complain of pulmonary distress for two weeks and above to the general hospitals. A total of six hundred (600) patients below 80 years of age were screened and enrolled for this study. The percentage prevalence of the physical appearance the sputa revealed that muco-purulent (20.0%), purulent (10.8%), mucoid (14.2%), and salivary (46.6%). The study revealed that 25.5% (153/600) of the studied participants were positive for Mycobacterium tuberculosis. The most affected age group was between 11-40 years with the percentage prevalence of 81.6% compared with the age group between 41-0 years. Male Patients had a higher prevalence at 60.1%, compared to 39.9% in females. The statistical analysis revealed that there was a significant difference with respect to gender and age (p < 0.05). The high prevalence of PTB among the subjects is greatly concerned by public health and it can be partially attributed to the poor socio-economic factor, overcrowding, social habits, and unavailability of clean portable water and lack of adequate sanitary condition.
Keywords: Prevalence, Pulmonary, Tuberculosis, Microscopic and Culture
Cite this paper: Sani R. A. , Garba S. A. , Oyeleke S. B. , Abalaka M. E. , Prevalence of Pulmonary Tuberculosis (PTB) in Minna and Suleja Niger State, Nigeria, American Journal of Medicine and Medical Sciences, Vol. 5 No. 6, 2015, pp. 287-291. doi: 10.5923/j.ajmms.20150506.04.
Article Outline
1. Introduction
- Tuberculosis is a chronic disease that affects man and other mammals, birds, fish and reptiles. Mammalian tuberculosis is caused by four very closely related species such as M. tuberculosis (the human tubercle bacillus), M. bovis (bovine tubercle bacillus), M. microti (the vole tubercle bacillus) and M. africanum rodent tubercle bacillus). These together formed Mycobacterium tuberculosis complex that causes infections and transmissible TB disease to human and other animals. Another mycobacterial species called None Tuberculosis Mycobacterium (NTM) are increasingly encountered as a major cause of disease in humans. These bacteria are also known as tubercle bacilli because they produce characteristic lesions called tubercles (WHO, 2013). The roles of its (Mycobacterium tuberculosis) complex, contribute to its virulence which is contained in the Mycobacterium cell wall. The cell wall’s main constituents are mycolylarabinogalactan, lipo-arabinomannan, and mycolic acids. Their three-dimensional distribution and the exact molecular mechanisms of the most effective anti-tuberculosis drugs that target the cell wall are not yet fully understood (Alsteens et al., 2007). According to World Health Organization global report 2014, that one third of the world’s population is infected with the bacilli that causes tuberculosis and the report collected for 2013, shows that there were an estimated 9.0 million new cases of tuberculosis (TB) globally, with most cases occurring in resource-limited or resource-poor countries in Asia (55%) and Africa (28.9%). Eighty percent of all cases worldwide occurred in the 22 high-burden countries with an estimated 1.5 million deaths occurring among all cases in 2013 and approximately 4105 deaths every day One of the most important factors influencing the current TB epidemic in resource-limited settings is poverty, which is closely related to malnutrition, crowded living conditions, lack of access to free or affordable health care services, and dependence on traditional healers that can facilitate the transmission of tuberculosis (WHO, 2014). The co-infections between TB and HIV have caused active disease of both infections and greatly increase their morbidity and mortality rate (WHO, 2014). Pulmonary tuberculosis (PTB) cases accounted for 84% of the total TB caseload and Extra Pulmonary Tuberculosis (EPTB) for 16%. Treatment outcomes have improved with new smear positive cure rates of 58% and treatment success rates of 71% in 2005 compared to rates of 51% and 66% respectively in 2004. The poor documentation of cure (high completion rates), defaulter rates over 10% and large numbers of unevaluated cases are indicative of poor systems at health facilities, and contribute to the failure to reach program targets. (National Tuberculosis Management Guidelines 2009). It is against this background that this study was designed to determine the prevalence of pulmonary Tuberculosis among children and adults attending out-patient department of the selected general hospitals.
2. Materials and Methods
2.1. Ethical Letter
- Ethical clearance was obtained from Niger State Hospitals Management Board for permission for the collection of samples from the two Hospitals Minna and Suleja.
2.2. Study Area
- The study areas include General Hospitals, Minna and Suleja, where tuberculosis cases are attended and treated promptly. These Hospitals have Directly Observed Treatment short course (DOTS) where TB Patients are attended to and receive treatment.Study Participant: Sixhundred participants attending out-patient department of Minna and Suleja General Hospital were encouraged and enrolled into this study. The enrolled participants were between the ages of 1-80 years of age. Sputum samples were collected in triplicate (spot, morning, spot) from all enrolled patients with the history of cough for more than 2 weeks and tested for tuberculosis. Positive samples were stored at 4°C for up to 3 days and transported to the Provincial Diagnostic Mycobacteriology Laboratory in Zaria (National Tuberculosis and Leprosy Training Centre Zaria) were the sputum was cultured.
2.3. Staining and Culturing of the Sputa
- The sputum samples were processed and decontaminated using standard microbiological procedure and were homogenized by shaking for 15 minutes with a wrist-action shaker. The supernatants were discarded and the deposit was rewashed by re-centrifugation. The final deposits were examined microscopically and cultured. The DOTS recommended technique was adopted for the staining procedure (Ziehl-Neelsen’s ZN staining procedure). This technique was adopted because of previous success rate of 80% (Cruickshank et al, 1975; Baker et al, 1998; Prescott et al, 2002).Lowenstein Jensen Glycerol Egg medium was used to culture the processed sputa. Results were read after 6-8 weeks and were regarded as negative if no colonies appeared after 8 weeks as earlier described by Idigbe and Onwujekwe, (1983). Cultures were considered positive for tuberculosis if they showed a positive growth on Lowenteine Jensen media and the presence of acid fast bacilli (AFB) by Ziehl-Neelsen (ZN) stain, and tested positive on a rapid TB antigen assay (SD-Bioline Ag MPT64 Rapid assay; Standard Diagnostics, Kyonggi-do, Korea). Cultures with growth on LJ media but negative on SD-Bioline were considered non-tuberculous mycobacterium isolates and were excluded from the research.
3. Results
- The results in figure 1; revealed that of 600 hundred Patients were screened for the research. 300 patients were screened from each hospital, 153(25.5%) patients were positive for TB while 447 were screened negative.
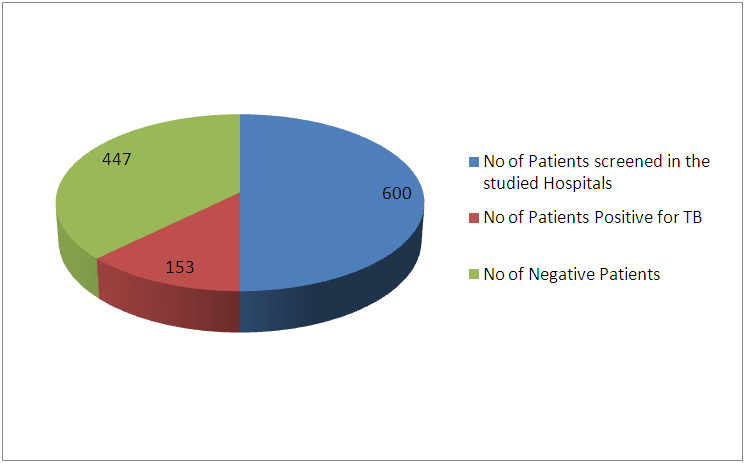 | Figure 1. Prevalence of Tuberculosis in Minna and Suleja |
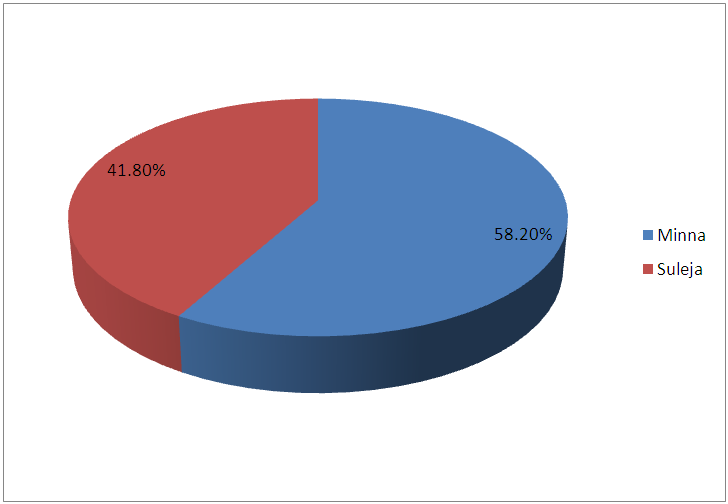 | Figure 2. Prevalence of tuberculosis in Minna and Suleja |
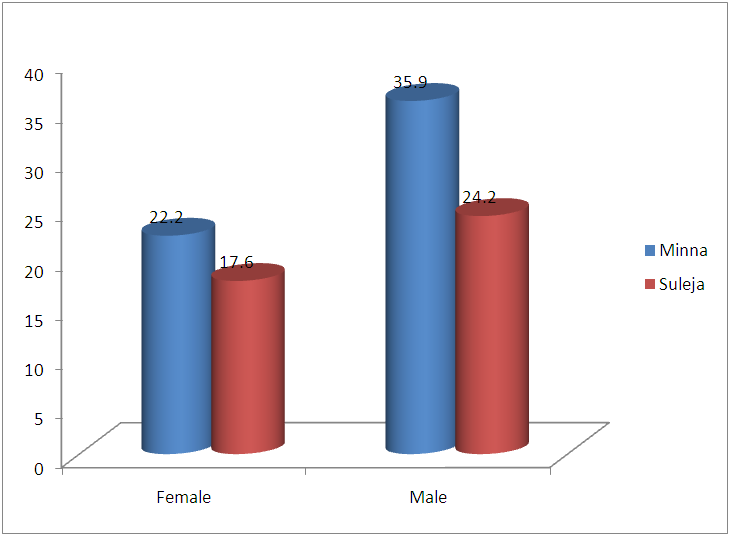 | Figure 3. Prevalence of TB based on Gender in Minna and Suleja |
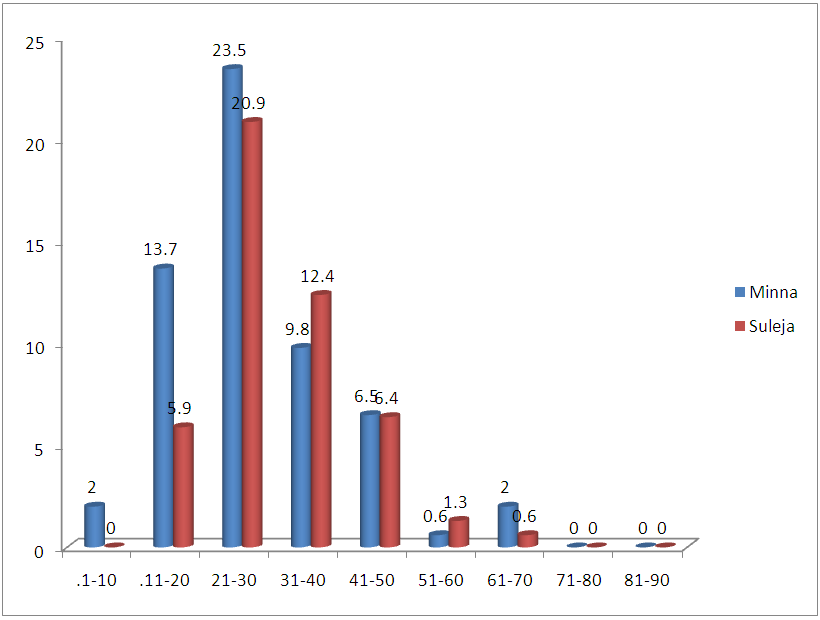 | Figure 4. Age distribution of Patients infected with tuberculosis in Minna and Suleja |
|
4. Discussion
- In this study, 600 Patients were enrolled and the prevalence of TB in this study areas is 25.5% and this is represented in figure 1and 2, and this in agreement with the study of Aliyu et.,al 2013 from Northern parts of Nigeria who found the prevalence of TB infected Patients to be 23.o% but our prevalence is higher than the prevalence of 6.9% recorded in the studies of Kehinde and Okesola who studied the Epidemiology of Clinical Isolates of Mycobacterium tuberculosis in Ibadan, Nigeria. The reasons for the high prevalence of Patients infected with TB fund from this study could be as a result that the study was carried out in the Hospital that serves as a referral sites for other smaller Hospitals who don’t have DOTS services. The highest percentage prevalence of tuberculosis was in Minna with 89 (58.2%) compared to that of Suleja 64 (41.7%). This may be due to urban migration, environmental factor, Tradition believes, in adherence to drug intake. Moreover, General Hospital Minna serves as the referrer center for all Patients in Niger and there is an higher influx of Patients than General Hospital Suleja. The prevalence in relation to gender as shown in Figure 3 shows that out of the 247 female that were screened, 186 (75.3%) were negative and 61 (24.6%) were positive for tuberculosis and out of 353 male that were screened, 92 (26.0%) were positive. Males were shown to have the highest prevalence of tuberculosis than the females. This could be as a result of behavioral attitude of males of this age group and in addition to this, higher prevalence could also be as a result of indiscriminate use of drug and its abuse. This study is in line with an earlier reported works done by Alfred and Silas (2005) in Uyo, AkwaIbom State, Obioma, et,.al (2011) in Port Harcourt, River State, Kehinde A.O, and Okesola in Ibadan Oyo State but this research is not in agreement with Nwachukwu, et,.al (2009) a research done in Abia State of Nigeria.The age group that has the highest prevalence of tuberculosis was age group eleven to fourty years (81.6%) compared with all other age groups, this is represented in figure 4. The high prevalence of tuberculosis among this age group could be as a result of increase in reproductive activities among this age group as they always refer to them as "reproductive age”. Higher prevalence of infection at this age group could also be attributed to increase in outdoor activities, overcrowding in most of the settlements and poor personal hygiene. This findings is in agreement with the research done by Nwachukwu, et.al., (2009) and Uzoewulu et.al., (2014), who reported that higher prevalence of tuberculosis infection lies between the age of twenty one to fourtyyears. While our study is not in agreement with the findings of (Okonko et.,al, 2012) whose study showed that TB infection was higher in age group 40years and above.Table 1 represents the physical appearance of the samples collected. 46.6% of the samples collected were salivary while the lowest percentages of samples were bloody (8.3%).In Conclusions, this Research shows higher prevalence of tuberculosis among Patients attending the studied hospitals. Prevalence among males was significantly higher than female patients also higher prevalence was recorded with patients of the age group 1-40. High prevalence of TB documented from this study areas of Niger State could be attributed to the fact that these two hospitals serves as a referrer hospitals to the people of these communities or due to overcrowded environment, method of buildings (compacted building) and lack of DOTS (Directly Observed Treatment Short Cost) in the rural areas surrounding the Local Government Areas.The transmission of TB in this areas can be reduced by educating the popular on the risk of TB transmission, early diagnosis and treatment, creation of DOTS services with qualified staff should be done in all the rural areas of the State, equipment for diagnosis of Extra Pulmonary TB (EPTB) and diagnosis of TB in children should be created by the State Governmentto argument the effort of the Non -Governmental Organization, Center for Disease Control, WHOetc. in fight against the spread infection.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML