-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(5): 246-252
doi:10.5923/j.ajmms.20150505.10
Serum Cystatin-C as an Early and Efficacious Biomarker of Diabetic Nephropathy in Renal Patients
Khalid Bassiouny, Hany Khalil, Wael S. Abed-Elmageed, Khalil A. El-Halfawy
Department of Molecular Biology, Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Egypt
Correspondence to: Khalil A. El-Halfawy, Department of Molecular Biology, Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Chronic kidney disease (CKD) is a major public health concern worldwide due to its rapid and random prevalence especially in diabetic patients. Further, diabetic nephropathy is a common cause of renal failure in diabetic patients accompanied with low levels of albumin in urine at early stage. In this study, we aim to investigate the possible utilizing of serum cystatin-C as an early and efficacious biomarker for renal function.Eighty five blood samples were collected from renal or diabetic patients with age variation and divided into three different groups, renal nondiabetic, diabetic nonrenal and healthy controls. Concentrations of related factors including serum creatinine and cystatin-C were investigated using the dimension clinical chemistry system. P values were statistically calculated for renal and diabetic related factors in addition to estimated glomerular filtration rate (eGFR). The blood samples obtained from renal or diabetic patients showed superior relationship between level of serum cystatin-C and eGFR rather than monitored values for creatinine. Moreover, our results showed a significant increase of serum cystatin-C in diabetic patients compared with normal individuals, indicating that serum cystatin-C is a crucial factor for early recognition of renal alternation in comparison with creatinine levels. The current data further confirm the connection of serum cystatin-C and GFR and indicate that serum cystatin-C is a predictive biomarker for CKD in diabetic patients.
Keywords: Renal disease, Diabetes, Early detection, Serum cystatin-C
Cite this paper: Khalid Bassiouny, Hany Khalil, Wael S. Abed-Elmageed, Khalil A. El-Halfawy, Serum Cystatin-C as an Early and Efficacious Biomarker of Diabetic Nephropathy in Renal Patients, American Journal of Medicine and Medical Sciences, Vol. 5 No. 5, 2015, pp. 246-252. doi: 10.5923/j.ajmms.20150505.10.
Article Outline
1. Introduction
- Renal failure is a medical condition in which the kidney loses the ability to filter the waste from blood. Renal failure consists of two major types: acute kidney injury (AKI) and chronic kidney disease (CKD). Usually, renal failure is determined by decrease in glomerular filtration rate (GFR) maintained by levels of creatinine in blood and blood urea nitrogen (BUN) [1-3]. AKI is abrupt loss of kidney function normally arises when kidneys are overloaded with cytotoxic compounds or when blood supply is suddenly interrupted. Probably, drug overdoses and chemotherapy cause the onset of AKI [4]. CKD is a long progressive loss of renal function caused by various medical conditions such as uncontrolled high blood pressure (hypertension), diabetes mellitus and polycystic kidney disease [1, 5]. Usually, hypertension and diabetes are the principal causes of CKD mainly by affecting blood vessels causing structural and functional alternations in the kidneys. Together with hypertension, diabetic patients suffer from high glomerular pressure resulting in stretching of capillary wall. The wall stretch mechanism occurs via different pathways including metabolic, hemodynamic and inflammatory pathway. Typically, the inflammatory pathway activates both gene expression and protein secretion of ICAM-1 and MCP-1 which promote an inflammatory cytokines production. Finally, these cytokines leads to glomerular injury and provides a further mechanism by which glomerular capillary hypertension may exert deleterious effects in diabetic glomerulopathy [6-8]. Such medical condition is a part of diabetic nephropathy or Kimmelstiel-Wilson Syndrome (KWS) in which kidney glomeruli and capillaries form angiopathy vessels leading to renal failure disorder [3, 5]. Notably, early recognition of glomerular nephropathy is very critical to successfully delay the advanced stages of renal disease. Therefore, different related parameters have been used to recognize renal disease including BUN level, urine creatinine and serum creatinine concentration which exploits for estimating GFR [9-11]. Indeed, creatinine can be accurately calculated by relative measurement in the blood and urine, subsequently GFR can be estimated by standard equations using the blood test results [12-14]. Recently, serum cystatin-C is produced at a constant rate by all nucleated cells and released into bloodstream. Cystatin-C, is freely filtered by kidney glomeruli, metabolized by the proximal tubule and identified as a promising marker of renal failure [15-17]. In the current study, we further investigated levels of serum cystatin-C in patients with either renal or diabetic condition taken in consideration sex and age variation. Findings strongly revealed the sensitive connection between serum cystatin-C and estimated GFR in renal patients. Interestingly, levels of serum cystatin-C were significantly increased in diabetic patients, including diabetic children, independent from renal failure indicating the beneficial role of serum cystatin-C as a predictive indicator for diabetic nephropathy in diabetic patients.
2. Materials and Methods
2.1. Sample Collection
- A total of 85 blood samples were obtained from patients with renal or diabetic disease in addition to healthy individuals (non-renal and non-diabetic patients) serving as control samples. To cover sex and age variations, 29 blood samples (15 males and 14 females) were collected from children with an average age of 5 years. 28 samples were obtained from adults (14 males and 14 females) with an average age of 30 years and 28 samples were obtained from patients (14 males and 14 females) with mean age of 55 years. Concentrations of related factors including glucose, creatinine, BUN, cystatin-C, and GFR were determined for each blood sample.
2.2. Laboratory Investigation
- The laboratory investigations of blood samples were performed using dimension clinical chemistry system (RxL Max. Siemens Healthcare Diagnostics). All blood samples were collected in serum separators tubes (SST) and incubated for 30 min to induce clotting. Serum creatinine was determined according to Jaffe reaction manufacture protocol [11]. Levels of glucose in blood samples were measured by using special sterilized tubes, followed by centrifugation. Isolated plasma was incubated with hexokinase-glucose-6-phosphate dehydrogenase substrate, then glucose concentrations were measured at wavelength of approximately, 340 nm. Glycated hemoglobin was monitored by collecting blood samples on EDTA and using turbidimetric inhibition immuno assay (TINIA). The measurement principle of this assay is depending on intensity of transmitted light on each sample and used for many commercial immunoassays. Relative concentration of cystatin-C was measured at wavelength of 450 nm using quantitative a sandwich immunoassay (Immunospec-USA Elisa kit). The micro-titer plats contain horseradish peroxidase (HRP)-conjugated polyclonal antibodies specific for cystatin-C. Finally, GFR was calculated dependent on creatinine concentration rate of each sample.
3. Staitististic
- Student-t test was used to calculate P values for each related factor. GFR values was calculated according to the equation, eGFR (mL/min/1.73 m2) = 186 X [creatinine (mg/dL)]-1.154 X (age)-0.203X 0.742 (for women) [9].
4. Results
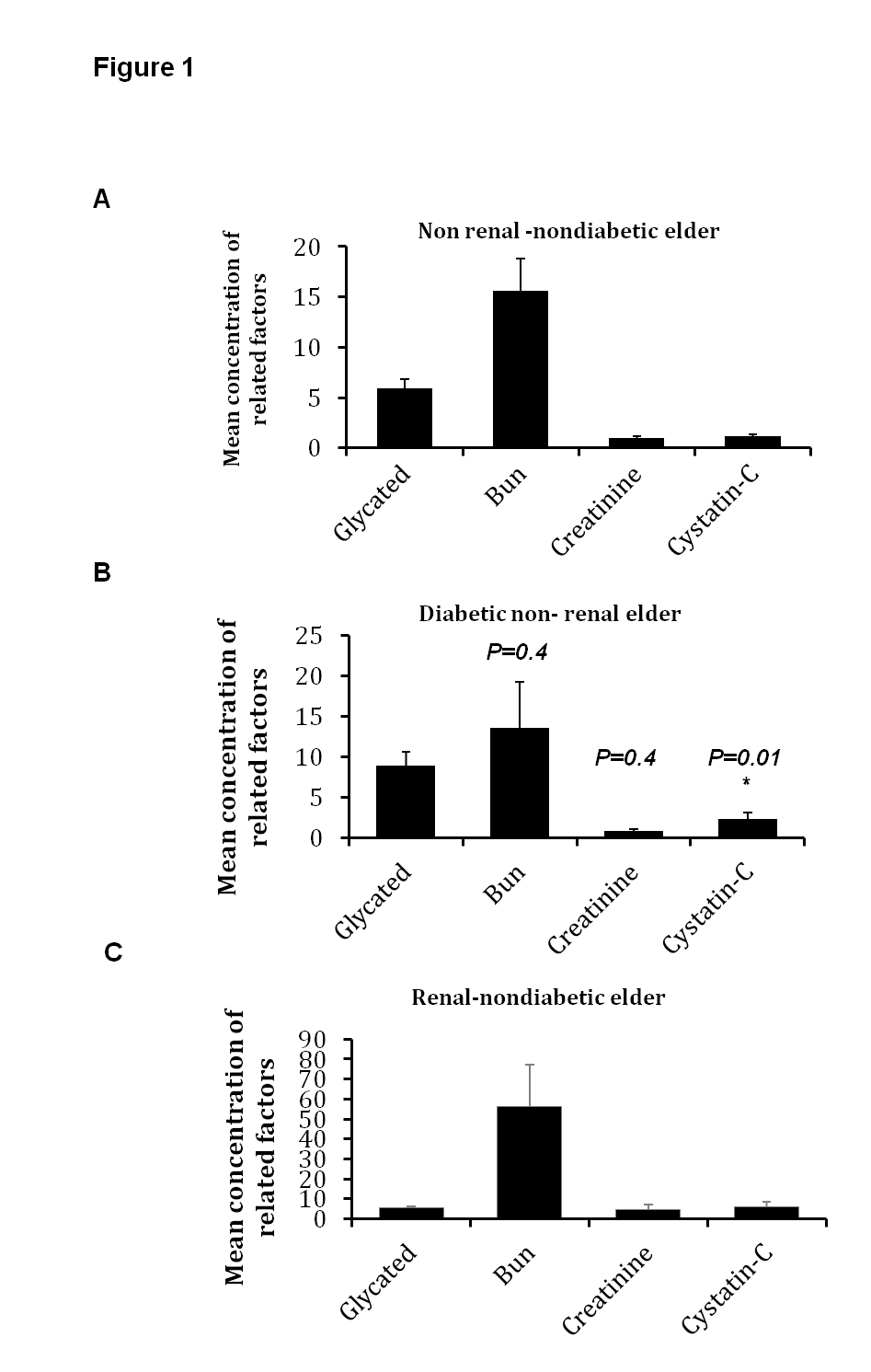
4.1. Level of Serum Cystatin-C is Increased in Elder Renal and Diabetic Patients
- In comparison with healthy individuals (non-renal and non-diabetic), the mean glucose level was 253 mg/dL in diabetic elder patients, which indicates diabetic condition in tested samples (Fig.1 A and B). The mean concentration of serum creatinine and BUN were 4.58 mg/dL and 56.4 mg/dL respectively. Interestingly, mean concentration of serum cystatin-C was up-regulated in renal elder patients (6.1 mg/dL), approximately 6 folds up-regulation in comparison with healthy controls (Fig.1 A and C). Furthermore, unlike other renal factors, including estimated GFR, levels of serum cystatin-C was significantly higher in diabetic elder patients compared with non-diabetic persons reported by statistical significance t test, P = 0.01 (Table 1). These results indicate that serum cystatin-C can be used as an effective biomarker and early indicator for renal failure in elder diabetic patients.
|
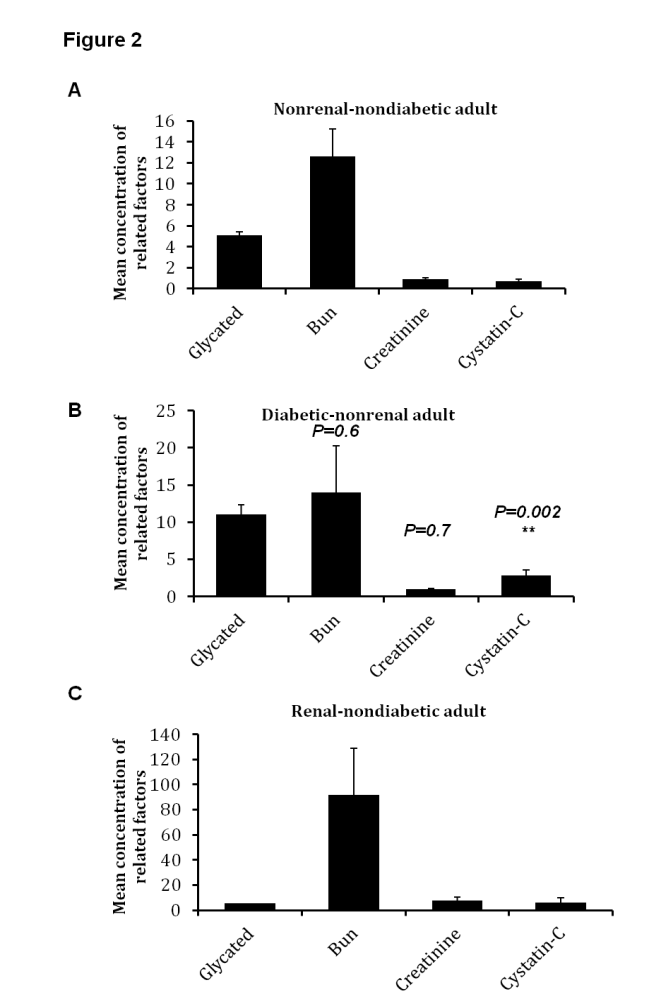
4.2. Concentration of Serum Cystatin-C is Increased in Diabetic and Renal Adult Patients
- To further validate our observation, serum cystatin-C and other renal factors were also investgated in adult patients with renal or diabitic disease. Levels of glucose and renal factors were significantly up-regulated in diabetic and renal patients, respectively (Fig. 2). Typically, mean concentration of serum cystatin-C was 2.6 mg/dL in renal adult patients while in normal individual, the mean concentration was 0.26 mg/dL (Fig. 2 A and C). Additionally, concentration of serum cystatin-C was significantly increased in diabetic patients in comparison with non-diabetic persons, P = 0.00023, whereas other indicated renal factors showed negligible variations between different samples (Table 2). These results further confirm the beneficial impact of serum cystatin-C in early detection of renal failure in both renal and diabetic patients.
|
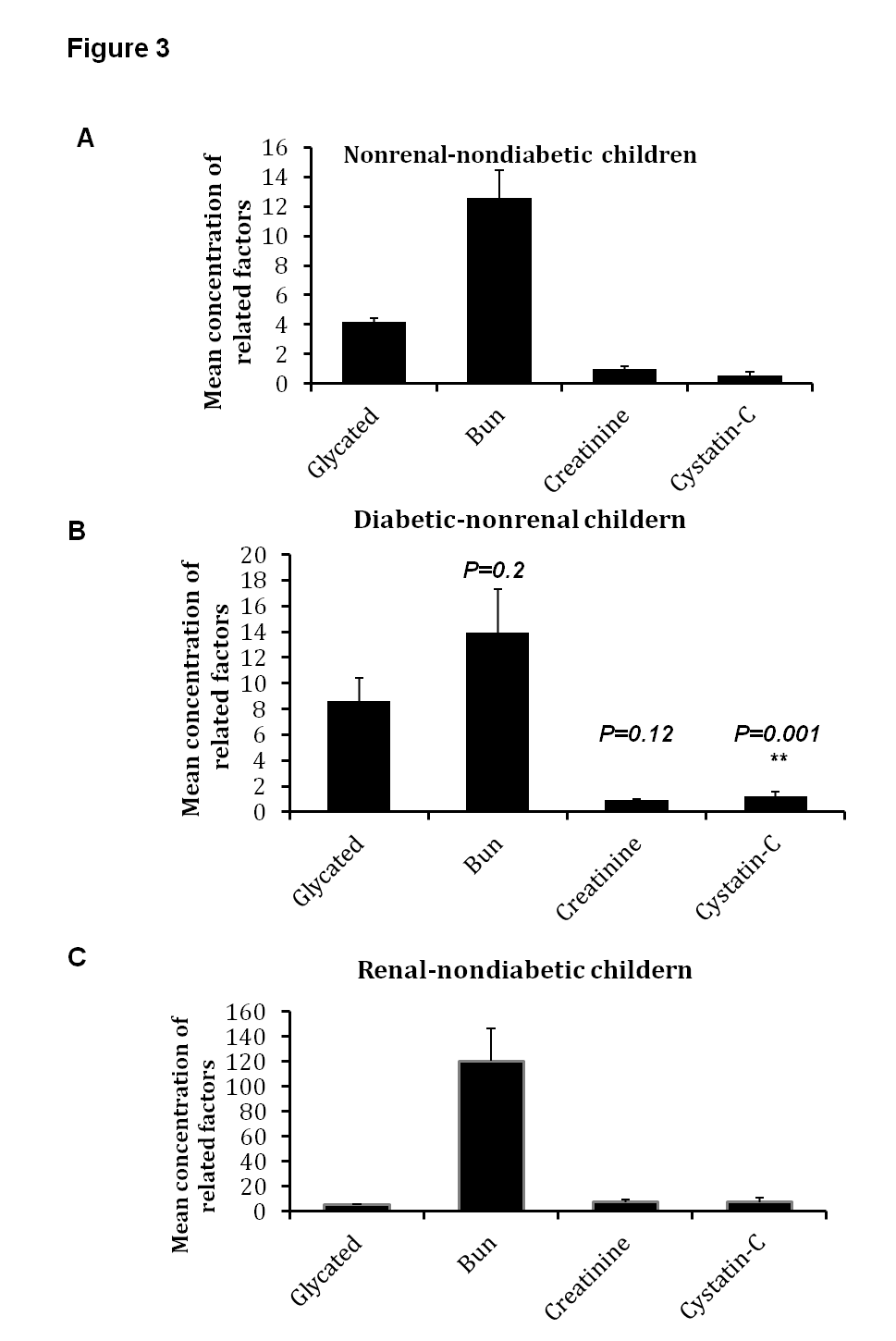
4.3. High Secretion of Serum Cystatin-C is Involved in Renal and Diabetic Children
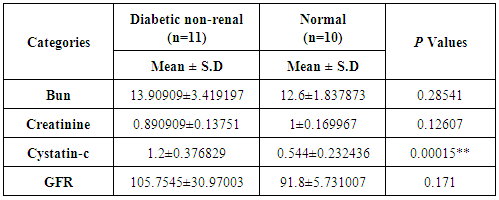
- In children with renal failure or diabetic condition, levels of serum cystatin-C were measured in addition to other related factors. Predictably, mean glucose levels were increased in diabetic children in comparison with renal and non-diabetic children, 314 mg/dl and 88.9mg/dL, respectively (Fig. 3 A and B). All renal related factors including creatinine and BUN were increased in adolescent samples, 7.3 mg/dL and 120 mg/dL, respectively (Fig. 3 C). Interestingly, the mean concentration of cystatin-C was increased in renal children (7.4 mg/dL) indicating 10 times more compared with non-renal children (0.7 mg/dL) (Fig. 3 A and C). Importantly, in diabetic non-renal children, the mean concentration of BUN and serum creatinine were in normal ranges (15.1 mg/dL BUN and 1.5 mg/dL creatinine) in comparison with non-diabetic children (12.1 mg/dL BUN and 1.1mg/dL creatinine). In contrast, the mean concentration of serum cystatin-C was increased from 0.5 mg/dL in healthy controls to 2.5 mg/dL in diabetic children. Additionally, the calculated P values showed a significant variation on cystatin-C in diabetic children compared with normal children reveals a value of 0.00015 (Table 3). These results exhibit the superior connection between renal function and concentration of serum cystatin-C in comparison with the concentration of serum creatinine in renal children and suggest that cystatin-C is a crucial factor in early diagnostic of renal failure in diabetic children.
|
5. Discussion
- The current data further confirms the superior connection between renal failure and secretion of serum cystatin-C compared with the old methods and suggests that level of serum cystatin-C is an effectual indicator for diabetic nephropathy. Generally, renal disease detection is based on GFR values, which is typically estimated by the concentration of serum creatinine. Notably, the normal concentration of serum cystatin-C is approximately 0.53-0.92 mg/dL regardless of sex and age variation. The interruption of GFR leads to high secretion of cystatin-C in blood and subsequently reveals the efficiency of renal function [15, 17, 18]. Based on the equation, estimated GFR = 186 X [creatinine (mg/dL)]-1.154 X (age)-0.203X 0.742 (for women), there is a factor must be added concocted with sex and age conditions. This means that sex and age variation have critical effects on estimated GFR, meanwhile the secretion of cystatin-C showed negligible differences regarding sex and age variation [15, 18]. Therefore, the correlation between glomerular filtration and levels of serum cystatin-C must be considered in diagnostic progress of renal disease and early detection of glomerular nephropathy. In the current study, GFR was calculated depending on serum creatinine concentrations according to equation indicated by Levey et al., 2006. Our findings showed approximately 10 times less GFR values in elder renal patients in comparison with normal and diabetic patients. In contrast, levels of serum cystatin-C showed approximately 10 times more in elder renal patients. Like cystatin-C, serum creatinine showed increasing levels in renal patients compared with normal and diabetic individual. Importantly, serum creatinine levels in renal children was about 2 times less than serum cystatin-C levels indicating the influence of age variation on serum creatinine levels, even in renal patients. This observation indicates that serum cystatin-C is a more consistent candidate than serum creatinine in the early recognition of renal disease, particularly in diabetic patients. Renal disease in children is a serious complication connected with long-term diabetes. Therefore, regular screening and controlling of diabetes might improve the ability of renal function [19-21]. Importantly, early detection of renal function in diabetic children is extremely helpful in diagnostic progress and identification of appropriate treatment that prevents or delays renal failure condition. Here, our findings indicate that serum cystatin-C is a critical factor that can reveal the efficiency of renal function. Moreover, level of serum cystatin-C was significantly increased in diabetic patients compared with normal persons. Taken together, these results further confirm the superior connection between GFR and serum cystatin-C in comparison with old methods and indicating the critical impact of serum cystatin-C in early diagnosis of renal failure mainly in diabetic patients.
6. Conclusions
- Eighty five blood samples were collected from renal or diabetic patients with age variation including elder, adult, and children. Concentration of serum creatinine was detected and subsequently GFR was estimated for each sample. Levels of serum cystatin-c was independently measured and compared with other renal related factors. The findings showed increasing levels of serum cystatin-c in renal failure patient in superior connection with renal estimated GFR and independent from glucose level in diabetic patients. Importantly, in children, mean values of serum cystatin-c was increased up to 10 fold in renal failure children in comparison with diabetic and normal children, whereas the main values of serum creatinine was increased about 5 fold. These data indicate that serum cystatin-c is more predictive biomarker in early recognition of renal failure than serum creatinine.
ACKNOWLEDGMENTS
- The authors thank the chemist Faten Kenawy, Department of Biochemistry, Misr-International Hospital, for her technical support. The authors also thank Kyle Caution, Department of Microbial Infection and Immunity, Ohio State University, USA, for expert assistance in preparing the manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML