-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(5): 223-226
doi:10.5923/j.ajmms.20150505.06
Search for Murine Mammary Tumor Virus-Like Sequences in Egyptian Females Breast Cancer
Amany M. Tawfik1, Bosat E. B. Kassem2, Amal Ayoub1, Mohamed A. Abd El-Baseer3, Laila Mossa4, Salwa E. Abdel Hamid1, Gad Y. Mekki2
1Department of Microbiology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of General Surgery, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3PhD Molecular Biology, Botany and Microbiology Department Faculty of Science, (for boys), Al-Azhar University, Cairo, Egypt
4Department of Pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Salwa E. Abdel Hamid, Department of Microbiology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
In 2012, 1.7 million women were diagnosed with breast cancer. Since the 2008 estimates, breast cancer incidence has increased by more than 20%, while mortality has increased by 14%. The identification of the causes of breast cancer is a crucial research issue for the development of effective prevention and treatment strategies.Over the pastdecades there has been an increasing interest in the possibility that human breast cancer may be caused by viral infections. In this study we searched for presence of mouse mammary tumour virus-like sequences in Egyptian breast cancer specimens. Unselected paraffin-embedded ninety blocks of paired carcinoma and normal breasts were tested for the presence of mouse mammary tumour virus-like envelope gene by PCR. None of the specimens was positive for the presence of mouse mammary tumour virus-like sequences.
Keywords: Breast cancer, MMTV, env gene, PCR, Egypt
Cite this paper: Amany M. Tawfik, Bosat E. B. Kassem, Amal Ayoub, Mohamed A. Abd El-Baseer, Laila Mossa, Salwa E. Abdel Hamid, Gad Y. Mekki, Search for Murine Mammary Tumor Virus-Like Sequences in Egyptian Females Breast Cancer, American Journal of Medicine and Medical Sciences, Vol. 5 No. 5, 2015, pp. 223-226. doi: 10.5923/j.ajmms.20150505.06.
1. Introduction
- Breast tumours have been noted since antiquity and were probably first described in the Edwin Smith surgical papyrus originating from Egypt at around 2,500 BC [1]. Breast cancer is the most common cause of cancer death among women (522,000 deaths in 2012) and the most frequently diagnosed cancer among women in 140 of 148 countries worldwide. It now represents one in four of all cancers in women [2]. Breast cancer is the second most common cancer in the world and by far, the most frequent cancer among women with an estimated 1.67 million new cancer cases diagnosed in 2012 (25% of all cancers) [3]. It is the most common cancer in women both in more and less developed regions with slightly more cases in less developed (883,000 cases) than in more developed (794,000) regions. Incidence rates vary nearly four-fold across the world regions, with rates ranging from 27 per 100,000 in Middle Africa and Eastern Asia to 96 in Western Europe [4]. In Egypt breast cancer is the most common cancer among women. The incidence in Lower, Middle, and Upper Egypt is 33.8%, 26.8% and 38.7% respectively [5].The etiology of most cancers is multifactorial. Breast cancer is known to be influenced by gender and age and by environmental factors e.g., radiation, hormones, and lifestyle. The presence of the mouse mammary tumor virus-like envelope (MMTV-like env) gene in a large portion of breast cancers [6] has been confirmed in many laboratories [7 8 9]. MMTV is a B-type oncornavirus of the retroviridae family, was first identified in the milk of breast-feeding mice [10]. There is an evidence that a human mammary tumor-like virus (HMTV-LV) exists in humans that is 95% homologous to MMTV at the env gene sequence [11]. MMTV-LV is distinguishable from human endogenous retroviruses (HERVs), which are viruses found in normal human DNA and accounts for 1% of an individual’s DNA [12]. The possibility of mouse to human transmission of the virus, based on the observation that worldwide, the incidence of breast cancer in a geographic area is proportional to the incidence of the mouse species Mus domesticus [13]. It is generally accepted that MMTV causes breast cancers in certain strains of mice and can be transmitted horizontally as an infectious particle containing viral DNA via breast-feeding or vertically in the germline [14]. The hypothesis that a retrovirus homologous to MMTV is involved in human breast cancers has resulted in renewed interest in the etiology of human breast cancer. A meta-analysis of published studies revealed a significantly increased risk for breast cancer development after MMTV-LV infection [15]. It appears that MMTV-LV infection could increase the risk of breast cancer. In addition, the prevalence of MMTV-LV is much higher in breast cancer patients from Western countries than Asian patients. The aim of this study was to search for presence of MMTV-LV in Egyptian female breast cancer.
2. Materials and Methods
- Unselected paraffin-embedded 90 sections of paired breast carcinoma and normal breast tissue were obtained from histopathology laboratory, Al-Zahraa Educational Hospital archival material. Ten μm sections were cut using a microtome. The blade was cleaned systematically with alcohol between each sample to prevent cross-contamination. All equipment used was wiped with 10% sodium hypochloride solution. Unselected paraffin-embedded sections of paired tumors and normal breasts were obtained from histopathology laboratory, Al-Zahraa Hospital archival material from women who had undergone surgery for breast cancer. Ten μm sections were cut using a microtome. The blade was cleaned systematically with alcohol between each sample to prevent cross-contamination. All equipment used was wiped with 10% sodium hypochloride solution. Paraffin was removed from tissues with two washes of xylene, supernatant removed, and the pellet washed in 100% ethanol. Viral DNA was extracted using QIAamp-DNA mini kit cat. No. 51304 (Qiagen, Hilden, Germany). The DNA quality was subsequently tested by amplification of a 268-bp fragment of the β-globin gene as an internal control. The product of the reaction was run on 2% agarose gel along with 100 bp DNA ladder. According to QIAGEN protocol (QIAamp-DNA mini kit- cat. No. 51304) tissues were resuspended in 100 μl of digestion buffer (150 mm NaCl, 15 mm Tris-HCl, 1 mm EDTA, and 0.1% SDS) with 5 μl of proteinase K (20 mg/ml), incubated at 55°C for 3 h, and then at 95°C for 10 min to inactivate the proteinase K. The DNA was precipitated in ethanol, and the supernatant was removed. The pellet was dried and resuspended in 20 μl of 10 mm Tris (pH 8)-1 mm EDTA buffer containing RNase, and the DNA concentration determined using a spectrophotometer. The DNA quality was subsequently tested by amplification of a 268-bp fragment of of the β-globin gene.DNA was amplified from two 10-μm sections following the conditions described in previous publications [8 9] using 2N and 3N primers [15], (2N, 5′-CCTACATCTGCCTGTGTTAC (positions 1386–1405); 3N, 5′-ATCTGTGGCATACCTAAAGG (positions 1640–1621) to amplify a 250-bp segment of the env gene. The product of the reaction was run on 2% agarose gel along with 50 bp DNA ladder.
3. Results
- PCR was performed on DNA extracted from normal breast and breast cancer specimens. None of the examined samples was positive for the 250 bp DNA sequences that are homologous to MMTV env gene sequences are not present in the studied Egyptian patients breast tumors and also in the normal control group. Figure (1) shows photograph of the ethidium bromide-stained gel of DNA ladder, positive control 250 bp and DNA extracted from specimens.
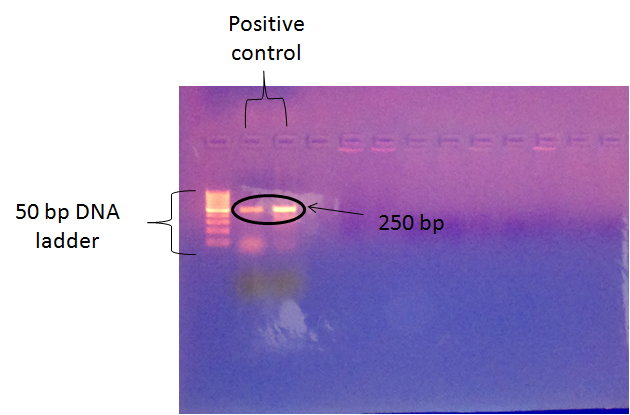 | Figure 1. Photograph of ethidium bromide-stained gel of PCR product |
4. Discussion
- Mouse mammary tumor virus (MMTV) is the etiological agent of mammary cancer in mice and it has long been postulated that this or a similar, MMTV-related, retrovirus is involved in breast cancer in humans [6, 16]. The aim of this study was to look for the presence of an exogenous MMTV-like envelope gene in breast cancer tissue from Egyptian females. We did not detect 250 bp DNA sequences that are homologous to MMTV env gene sequences in any of the studied breast cancer specimens and normal control group. Studies looking at the presence of viruses in breast cancer have produced conflicting results, and the possible association remains controversial. Despite a large number of molecular epidemiological studies, the association of MMTV-LV infection with the risk of human breast cancer remains inconclusive mainly due to the heterogeneity in populations [15, 17]. The reported prevalence of MMTV-LV infection in breast carcinoma samples varied geographically, and some researchers even reported their inability to detect MMTV-LV sequence using PCR in human breast cancer tissues. Our findings appear consistent with a growing number of independent negative reports internationally. Negative results were obtained by many authors [18-25] whom failed to detect MMTV-LV like sequences in female breast cancer specimens. These studies stimulated a new wave of controversy on the relevance of MMTV-LV to human breast cancer [15]. Many of recent papers have, however, suggested different interpretations for such discordant experimental findings. The inability to detect the virus may be due to the low number of copies of virus in the specimen that the concentration of a putative HMTV DNA is nil or very low in human malignant and non-malignant breast tissue [21].It seems possible that some negative results were the consequence of the low quantity of env sequences and/or technical and methodological problems.ISI Web of Science In their study, Kamal et al [26], investigated presence of MMTV-like gene sequence in archival 23 paraffin- embedded sections of malignant breast tumors and 10 paraffin-embedded sections of human benign breast tissue from Egyptian women. They detected 250 bp env gene-like sequence in 3 out of 23 breast cancer biopsies but could not detect 660 bp env gene-like sequence of the virus. The discrepancy between previous studies and the present study may arise from differing geographic distribution of etiologic agents, vectors, or clustering of populations with different infection of cancer susceptibilities, or may result from different MMTV diagnostic methods [23]. Some studies of Australian, Japanese and Chinese women revealed no association between MMTV-related retrovirus and breast carcinogenesis [22, 11]. The absence of MMTV DNA in both breast cancer samples and controls indicates either that the concentration of putative MMTV or HMTV DNA in the breast cancers was too low for detection or that it did not exist there.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML