-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(5): 204-207
doi:10.5923/j.ajmms.20150505.03
The Role of Interleukin (IL)-22 as a Pro-Inflammatory Cytokine in Systemic Lupus Erythematosus
Gehan H. Ewieda1, Omnia A. El-Dydamoni2, Nashwa El-Khouly3, 4
1Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of Microbiology & Immunology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3Department of Internal Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
4Department of Internal Medicine, Faculty of Medicine, Taibah University, KSA
Correspondence to: Gehan H. Ewieda, Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Background: Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by multiple organ damage in addition to amounts of autoantibodies generated by excessively activated B cells, abnormal T cell subsets and related cytokines are also involved in the pathophysiology of SLE. Interleukin (IL)-22 is a cytokine that is important for the modulation of tissue responses during inflammation. IL-22 has both pro-inflammatory and tissue-protective properties, therefore this study aimed to assess the role ofIL-22 levels in plasma of patients with SLE and compare it with normal controls. Also to correlate plasma levels of IL-22 with disease activity using systemic lupus erythematosus score (SLEDAI) in SLE patients.Subjects and Methods: Thirty five SLE patients (Group I) were subjected to full history taking and clinical examination, assessment of SLEDAI, laboratory investigations in the form of serological markers for systemic lupus activity, including anti-double stranded deoxyribonucleic acid (anti-ds DNA), anti-nuclear antibodies (ANA), and autoimmune profile complement components C3 and C4 levels, CBC, ESR, ALT, AST, blood urea nitrogen (BUN), serum and urinary creatinine and urinary protein, in addition to 40 age and sex matched healthy controls (Group II). Plasma IL-22 level using ELISA technique was done to all participants. Results:The plasma levels of IL-22 in SLE patients were significantly higher than normal controls with evidence of positive correlation between IL-22 levels and SLEDAI score. Conclusively, it could be suggested that the IL-22 may be implicated in the pathogenesis of SLE disease.
Keywords: Systemic Lupus Erythematosus Disease activity Index (SLEDAI)–Pro-inflammatory–Interleukin (IL)-22–Anti-double stranded deoxyribonucleic acid (anti-ds DNA), Anti-nuclear antibodies (ANA)
Cite this paper: Gehan H. Ewieda, Omnia A. El-Dydamoni, Nashwa El-Khouly, The Role of Interleukin (IL)-22 as a Pro-Inflammatory Cytokine in Systemic Lupus Erythematosus, American Journal of Medicine and Medical Sciences, Vol. 5 No. 5, 2015, pp. 204-207. doi: 10.5923/j.ajmms.20150505.03.
Article Outline
1. Introduction
- Systemic Lupus Erythematosus (SLE) is a chronic systemic disorder characterized by the development of an immune response directed against many body cells [1, 2]. SLE can affect the skin, joints, kidney, heart and other organs [3]. The etiology is still unknown but it is believed that genetic, immunological and environmental factors are involved in the pathogenesis of this disease [4]. In lupus, the body's immune system turns against antigens in the body's own nuclei, with activated B-cells producing antibodies against self DNA and associated proteins. The resulting immune complexes accumulate in the body, causing inflammation, tissue damage and organ failure. Immune cells from patients with SLE display many abnormalities, including reduced T cell cytotoxicity, abnormal function of T cells, abnormal activation of B cells, and alterations in cytokine biosynthesis [5-7]. Many inflammatory cytokines and chemokines are involved in the pathogenesis of autoimmune diseases [8, 9]. Interleukin 22 (IL-22) is a regulator of autoimmune diseases [10]. It is a novel cytokine which is a member of the IL-10 cytokine family and plays critical roles in inflammation, immune surveillance, and tissue homeostasis at mucosal sites [6, 11, and 12]. The human IL-22 gene is located on the longer arm of chromosome 12, on 12q15. It encodes a protein of 179 amino acids in length, which, after splitting off the signal peptide (33 aa), is secreted as a polypeptide of 146 amino acids [13]. Like all other IL-10 family members, IL-22 has a helical structure. IL-22 is an alpha-helical cytokine. It binds to a heterodimeric cell surface receptor composed of IL-22R1 and IL-10R2 subunits [14]. IL-22R1 is expressed in tissue cells, and is absent on immune cells. IL-22 is differentially expressed in many autoimmune diseases. It has not been determined whether IL-22 is mediating the inflammation itself, or is a by-product of the inflammation [15, 16]. IL-22 has also a protective role in inflammation. The dual nature of this cytokine, protective versus inflammatory, likely depends on the inflammatory context, which includes, but is not limited to, the duration and amount of IL-22 present [15-17]. The main objective of this work is to measure the levels of plasma IL-22 in SLE and to correlate plasma IL-22 levels with the disease activity.
2. Subjects and Methods
2.1. Subjects
- The current study included thirty five consecutive SLE patients were attending the outpatient clinic of Internal Medicine Department of Al-Zaharaa University Hospital, Cairo, Egypt, from March 2014 to December 2014 and aged between 16 to 49 years old served as group (I). They were 29 females and 6 males. The disease duration in all patients ranged from 1 to 12 years. In addition to forty healthy subjects' sex and age matched to the patients as control group (II), including 35 females and 5 males, their age ranged from 20-51 years. A verbal informed consent was obtained from all subjects enrolled in the study. All Patients were diagnosed according to the American College of Rheumatology (ACR) diagnostic criteria. Disease activity of SLE patients was assessed by Systemic Lupus Erythematosus Disease activity Index (SLEDAI). The SLE disease severity was assessed using the Systemic Lupus International Collaborative clinics/America Collage of Rheumatology (SLICC/ACR) damage index: the SCLICC/ACR damage index is a measurement of cumulative end organ damage in SLE. Damage is described as non-reversible change, not related to active inflammation, occurring since the onset of lupus, ascertained by clinical assessment. Patients with diabetes mellitus, malignancies and those with a diagnosis of mixed connective tissue disease were excluded.Sample collection: 4 ml venous blood were withdrawn in EDTA then centrifuged for 15 minutes at 1000 rpm within 30 minutes of collection. Plasma was removed in aliquot and stored at -20°C. Repeated freeze-thaw cycles were avoided.
2.2. Methods
- All patients and controls were subjected to complete history taking, full clinical and routine laboratory assessments in the form of serological markers for systemic lupus activity, including anti-ds DNA, ANA, and autoimmune profile complement components C3 and C4 levels, CBC, ESR, ALT, AST, BUN, serum and urinary creatinine and urinary protein.
2.3. Quantitative Detection the Levels of IL-22
- The levels of IL-22 in plasma were measured by enzyme- linked immunosorbent assay technique using commercially available human IL-22 ELISA kit, Catalog no.:RBMS2047R BioVendor, Asheville, NC 28806 USA, according to the manufacturer’s instructions.
2.4. Statistical Analysis
- Data were collected, revised, coded and entered to the statistical package for social science (SPSS) version 20. Numerical data were expressed as mean ± standard deviation (SD) and range. The Comparison between means of the two groups was done by using student's t-test. Spearman correlation co-efficient test was used to rank variables versus each other positively or inversely. The confidence interval was set to 95% and the margin of error accepted was adjusted to 5%. So, probability value (P-value) was considered insignificant at the level of more than 0.05, significant at the level of less than 0.05 and highly significant at the level of less than 0.01.
3. Results
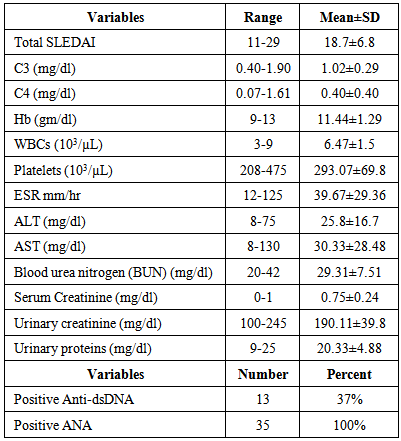
- As regard the results of the disease activity scores and laboratory parameters of SLE patients were shown in table 1.
|
|
|
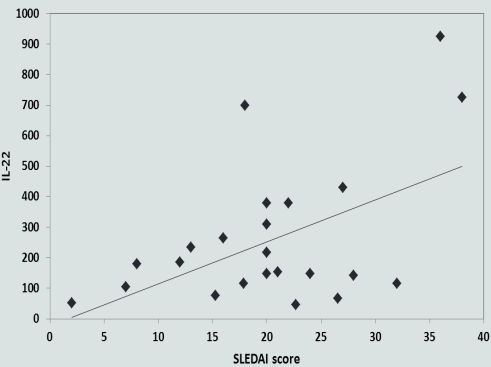 | Figure 1. Correlation between IL-22 level and disease activity index (SLEDAI) |
4. Discussion
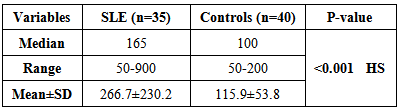
- In the present study, as regard the results of the plasma levels of IL-22 in SLE patients were significantly higher when compared to healthy controls (P≤0.001). This finding was in agreement with the study done by Zhao et al. [11]. They showed that significantly increased levels of plasma IL-22 in SLE patients than in normal controls (P = 0.0039). Results of our study were in accordance with the study done by Qin et al. [18], who found that the percentages of IL-22-positive CD4+T cells were increased in the peripheral blood mononuclear cells (PBMCs) of patients with SLE compared with healthy control subjects.On contrary to our results, significantly lower concentrations of plasma IL-22 in SLE patients than in healthy controls were observed by Zhang et al. [19]. Also in disagreement with our study, a recent study done by Lin et al. [7], who noticed that the plasma IL-22 levels in new-onset and relapsing SLE patients were lower than those in healthy controls. Additionally, plasma IL-22 levels in new-onset patients were significantly lower than in relapsing SLE patients and healthy controls. On the other hand the study done by Yang et al. [2], clinical data were collected in 65 SLE patients and 30 healthy controls. The patients were divided into active and inactive groups. They results show that the serum IL-22 was unchanged in SLE, compared with healthy controls (P=0.725).In the present study, we provided an evidence that positive correlation was found between IL-22 levels and SLEDAI score (r=0.498). Concomitant with our results, Zhao et al. [11] conducted on a total of 22 patients with freshly diagnosed SLE and 18 age-/gender-matched healthy controls (n = 18), and they showed that IL-22 +CD4+ T-cells were correlated positively with the values of SLEDAI in SLE patients. These data were also in consistent with Duhen et al. [20] and Qin et al. [18]. In contrary to our result, Yang et al. [2] showed that the serum level of IL-22 was not correlated with SLEDAI (P=0.282) in SLE patients, this result was also matched with Pan et al. [21]. Cheng et al. [22] showed that plasma IL-22 levels in all patients negatively correlated with SLEDAI and this was not in harmony to our results. Also, Lin et al. [7] noticed that plasma IL-22 levels negatively correlated with SLEDAI scores (r = _ 0.384, P = 0.003), this study targeted the new onset SLE patients and they were not receiving treatments.In the current study, plasma levels of IL-22 was positively correlated with ESR values (r=0.388, p=0.037). Our results in disagreement with the study done by Lin et al. [7], they found that the plasma IL-22 levels were negatively correlated with ESR values (r = _ 0.519, P = 0.023). On the other hand, Zhao et al. [11] showed that IL-22+CD4+ T-cells were not correlated with the values of ESR in SLE.In this study, there was no significant correlation between plasma IL-22 level and complement levels. Matching to our results, Zhao et al. [11] demonstrated that the IL-22CD4 T-cells was not correlated with complement level (P=0.05). Also in concomitant with our results, Lin et al. [7] showed that no significant correlation between plasma IL-22 level and complement level. The discrepancies between our results and others concerning SLE patients can be referred to our small sample size, varying disease severities, durations, and the different stages of the disease as well as therapeutic status as it is possible that there are dynamic changes in the levels of plasma IL-22 during the pathogenic process of SLE. The levels of plasma IL-22 in SLE patients may start at a high level and decline with the disease progression or after standard treatment or vice versa. Genetic considerations between our populations and others may also play a role. A study by Yu et al. [23], who reported that genetic variations in IL-22 copy numbers contribute to the risk of SLE, implicating that IL-22 in SLE risk might help to identify new targets for therapy. Genetic studying may help to define populations with high susceptibility to SLE.
5. Conclusions
- Conclusively, the plasma levels of IL-22 in SLE patients were significantly higher than normal controls. Importantly, a positive association between IL-22 and SLEDAI score and ESR suggesting that the pro-inflammatory cytokine may be implicated in the pathogenesis of this disease. We recommend that further studies should involve larger sample size of SLE patients, aiming to increase the awareness about the exact mechanism.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML