Rabie Fathy Abbas1, Ibrahim Aly Ibrahim2
1Internal Medicine Department, Faculty of Medicine (Boys), Al-Azhar University, Cairo, Egypt
2Clinical Pathology Department, Faculty of Medicine (Boys), Al-Azhar University, Cairo, Egypt
Correspondence to: Rabie Fathy Abbas, Internal Medicine Department, Faculty of Medicine (Boys), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Background and aim of the work: There is increasing interest in the role of vitamin D in inflammatory bowel disease(IBD),outside of its traditional role in metabolic bone disease. Novel insights into additional roles for vitamin D are being established and these include anti-inflammatory and immune modulating effects. Therefore, this study was planned to assess the level of vitamin D variations among ulcerative colitis patients in Egypt. After departmental ethical committee approval and participants consent were obtained Twenty(20) patients with ulcerative colitis(UC) (group I), and Ten(10) apparently healthy persons (group II) as control group were enrolled in the study. Ulcerative colitis patients was diagnosed through, full history taking, clinical assessment, laboratory investigations, colonoscopic and histopathological studies. The control group was subjected to diagnostic modalities aspatients group apart from Colonoscopic and histopathological studies, and matched with patients group for age and sex. Group (1) ages ranged from 21 – 60 years, mean age was (36.95) 13 were females (65%) and 7 were males (35%). Group (11) ages ranged from 20 -58 years, mean age was (37.60) 6 were females(60%) and 4 were males (40%). Results of this study revealed: The mean serum values of 25(OH) vitamin D in patients group(I) were lower in comparison to control group (II) (23.0±13.49 ng/dl and 36.2±5.9 ng/dl) respectively. This difference was statistically significant (P<0.007). Laboratory data of studied groups showed statistically significant difference between controls and patients group regarding HB, C-reactive protein, and serum calcium levels (P-value 0.000) respectively, as there were decreased level of HB and serum calcium in patients group compared to controls, however, increased CRP levels in patients group. White blood cells (WBCs) was higher in patients group compared to controls. In ulcerative colitis patients, there were inverse correlation between frequency of bloody stools/day and CRP with vitamin D levels which statistically significant (P= 0.003, 0.000) respectively. Serum calcium and vitamin D levels were in direct correlation and statistically significant (P=0.000). There were no correlation between age, WBCs, platelets, s. albumin, s. creatinine and INR with vitamin D levels. The patient group was classified into 3 subgroups: deficiency subgroup (9 patients) with main serum values of 25(OH) vitamin D (14.4±2.86 ng/ml), insufficiency subgroup (8 patients) (24.19±2.76 ng/ml) and sufficiency subgroup(3 patients) (33.67±3.06 ng/ml.). There was an evidence of exacerbation of activity of ulcerative colitis correlated to the degree of decreasing of vitamin D level. There were significant increase in frequency of bloody stool motions/day, ESR, and C-reactive protein (CRP), with the degree of decrease of vitamin D levels in patients subgroups. However, there were decrease of haemoglobin, serum phosphate & calcium with the degree of lowered vitamin D level in patients with ulcerative colitis. Conclusions: vitamin D [25(OH) D] deficiency is common in ulcerative colitis patients, the increased the degree of vitamin D deficiency, is associated with the increased the degree of activity of ulcerative colitis disease, which may support suggestion of vitamin D level is a potential contributing factor underlying the pathogenesis and disease activity of ulcerative colitis.
Keywords:
IBD, Ulcerative Colitis, Ulcerative colitis activity, Vitamin D status
Cite this paper: Rabie Fathy Abbas, Ibrahim Aly Ibrahim, Study of Vitamin D Status in Patients with Ulcerative Colitis in Egypt, American Journal of Medicine and Medical Sciences, Vol. 5 No. 4, 2015, pp. 168-174. doi: 10.5923/j.ajmms.20150504.05.
1. Introduction
Inflammatory bowel disease (IBD), comprising crohn’s disease (CD) and ulcerative colitis (UC), is a group of debilitating conditions affect up to 0.5% of population in developed countries and an increasing proportion in developing nations [6]. The pathogenesis of IBD is thought to involve a complex interplay between genetic, environmental, and microbial environments in the context of an inappropriate and abnormal activation of the mucosal immune system, Particularly adysregulated intestinal mucosal T –cell mediated immune response, specifically CD4T helper type-1(Th 1) lymphocytes, leads to production of Th 1-associated pro-inflammatory cytokines, such as interferon-ɣ (IFN-ɣ) and tumour necrosis factor-alpha (TNF- α). Another potential pathogenic factor in CD is impaired mucosal barrier function and intestinal hyperpermeability [11]. Patients with UC will typically experience periods of relapse follow periods of remission, lasting months to years [7]. During relapses, symptoms of abdominal pain, diarrhoea and rectal bleeding worsen. During remissions, these symptoms subside. Remissions usually occur because of treatment with medications or surgery, but occasionally they occur spontaneously, that is, without any treatment [13]. Vitamin D deficiency is more common in adults and children with Crohn’s disease than healthy controls and correlates with poorer health-related quality of life and greater disease activity [15]. It is not known whether a low vitamin D status is acontributing factor and / or a consequence of IBD [14]. Some non-traditional roles ascribed to vitamin D include anti-inflammatory and immune-modulating effects. These effects have led to possible implications in the pathophysiology of immune-mediated diseases including multiple sclerosis and IBD [10]. Vitamin D has been recognized as essential for the normal development and mineralization of a healthy skeleton. However, the discovery of vitamin D receptor in most tissues and cells in the body has provided new insights into the function of this vitamin. There has been a dramatic increase in research examining the role of vitamin D and its receptor in many chronic illnesses, including cancers, cardiovascular diseases, and autoimmune diseases such as multiple sclerosis and IBD [12]. There is increasing interest in the role of vitamin D in IBD, outside of its traditional role in metabolic bone disease. Novel insights into additional roles for vitamin D are being established and these include anti-inflammatory and immune modulating effects. Active vitamin D is known to exert its biological functions via the vitamin D receptor (VDR). Immune cells have been found to express VDR and possess the enzymes necessary to produce active vitamin D. This suggests vitamin D may have actions beyond endocrine activity [3].
2. Patients and Methods
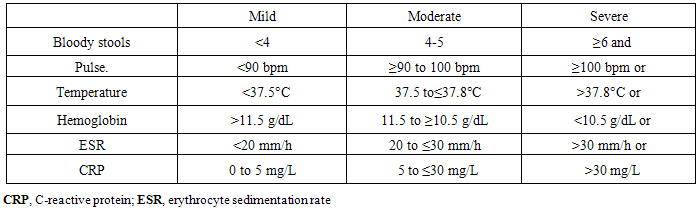
Patients: After departmental ethics committee approval and all participants consent were obtained, twenty (20) patients with ulcerative colitis as group (I) and ten (10) apparently healthy persons as control group (II) were enrolled in this study over a period from 10/2013 to 10/2014. Ulcerative colitis patients (group I) and controls (group II) were selected from outpatients and inpatients of internal medicine department at Sayed-Galal AL-Azhar university hospital. Ulcerative colitis patients were diagnosed, through clinical evaluation that suggested high possibility of ulcerative colitis (UC) as diarrhoea, bloody stool with mucus, tenesmus, fever and weight loss. Colonoscopic and histopathological studies were done, that confirmed the diagnosis of ulcerative colitis. Exclusion criteria: patients known to have malignancy, or colonic surgery, or metabolic disease associated with vitamin D and calcium abnormalities e.g. hyperparathyroidism and history of vitamin D supplementations. Patients with known biliary disease, chronic liver and kidney diseases. Patients with a mal-absorption syndrome other than IBD or on continuous doses of oral corticosteroids or short history of intake. Pregnant or lactating patients.Methods: All participants in this study were subjected to : Detailed history taking, full clinical examination, and laboratory investigations; stool analysis, liver function tests (alanine-amino-trasferase, serum albumin, international normalization ratio(INR).), serum creatinine, complete blood count (CBC), haemoglobin (HB), white blood cells (WBCs), platelets, erythrocyte sedimentation rate(ESR), C- reactive protein (CRP), fasting blood glucose, serum calcium and phosphate, all mentioned investigations were done to help in selection of patients, control group, and in assessment of the degree of ulcerative colitis disease activity, according to reported criteria of ulcerative colitis activity by Truelove and Witts [19]. AS SHOWN IN TABLE (1).Table 1. Disease Activity in UC
 |
| |
|
Quantitative measurement of serum total 25(OH) vitamin D by ELISA. Fasting (6-8 hours), venous blood samples(10ml) were collected from participants in the morning and after centrifugation, the serum was preserved in the deep freezer at -20 c. Serum levels were determined by using commercially available kits, DRG 25-OH vitamin D (total) ELISA (EIA-5396), DRG International, Inc., USA. According to Dumitrescu et. al [8], vitamin D level(vitamin D status) may be one of the following: a-Normal vitamin D : serum 25 (OH) D:> 30 ng/dl b- vitamin D insufficiency: serum 25 (OH) D: 20-30 ng/dl c-vitamin D deficiency: serum 25(OH) D: <20 ng/dl. The patients group were classified according to vitamin D levels to three (3) subgroups.Pelvi-abdominal ultrasound (US) was done to all participants by using, GE, Logic C2 machine.Total colonoscopy was performed to patients group (I) with exclusion of normal or non-specific colitis, and to help in assessment the severity of ulcerative colitis.Histo-pathological studies were done for all biopsies taken by colonoscopy to confirm the diagnosis of ulcerative colitis, with exclusion of cases not consistent with documented histopathological findings of the disease.Statistical analysis: analysis of the obtained data was done by IBM computer using SPSS(statistical program for social science version 12); description of quantitative variables as Mean, SD and range, description of qualitative variables as number and percentage, chi-square test was used to compare qualitative variables between both groups, unpaired-t-test was used to compare quantitative variables between both groups, correlation co-efficient test was used to rank different variables against each other, P value >0.05 insignificant P value< 0.05 significant, P value <0.001 highly significant.
3. Results
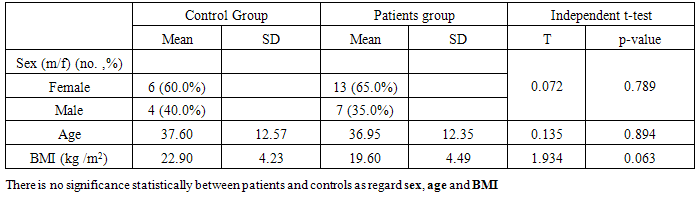
Twenty (20) patients with ulcerative colitis (UC) (group I) and ten (10) healthy persons matched for age and sex as control (group II) were involved in this study. Group (I) ages ranged from 21-60 yrs, mean age (36.9), 13 patients were females (65%) and 7 patients were males (35%). Group II ages ranged from 20-58 yrs, mean age was (37.6), 6 patients were females (60%) and 4 patients were males (40%).Table (2). Distribution of demographic data of patients and control groups
 |
| |
|
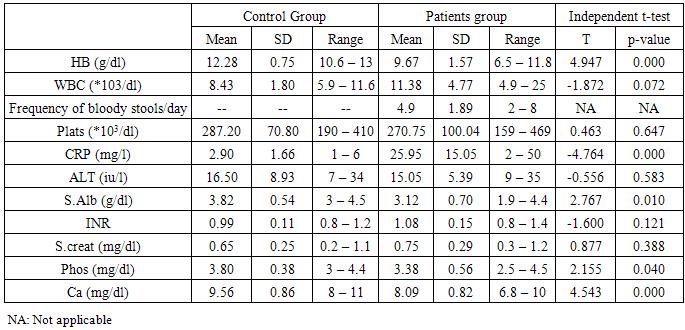
Table (3). Comparison between patients and controls regarding laboratory data
 |
| |
|
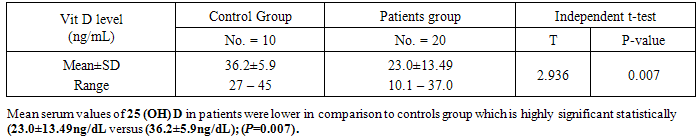
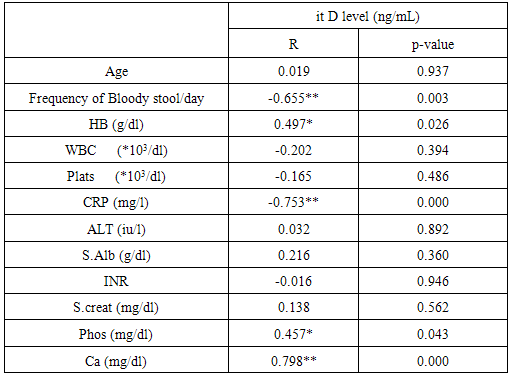
Analysis of laboratory data of the studied groups statistically showed highly significant difference between controls and patients group regarding, HB, C-reactive protein, and serum calcium levels (P value = 0.000) respectively, as, we noticed that there were decreased levels of HB, and serum calcium in patients group compared to controls, however, increased CRP levels in patients group. Also, slight decrease of serum albumin and phosphate in patients group in comparison to controls group, there was no statistically difference between both groups regarding other laboratory data. White blood cells (WBCs) was higher in patients group compared to controls group.Analysis of the obtained results as shown from table(5) revealed that, in ulcerative colitis patients, there were inverse correlation between frequency of bloody stool /day and CRP with vitamin D levels which is statistically highly significant (p=o.oo3, o.ooo) respectively. Also, ESR readings of this study were consistent with CRP levels, which might reflect ulcerative colitis disease activity ,associated with more lower levels of vitamin D. Serum Ca++ and vitamin D were in direct correlation and statistically highly significant (p=0.000). There were no significant correlation between age, WBCs, platelets, s. albumin, s. creatinine and INR with vitamin D levels.Table (4). Comparison between patients and controls regarding vitamin D levels
 |
| |
|
Table (5). Correlation between vitamin D level and other parameters in patients group
 |
| |
|
According to vitamin D status (levels) in ulcerative colitis patients, they stratified to 3 subgroups: deficiency subgroup (45%), insufficiency subgroup (40%), sufficiency subgroup (15%), as shown in figure (1), (Dumitrescu, et. al [8]).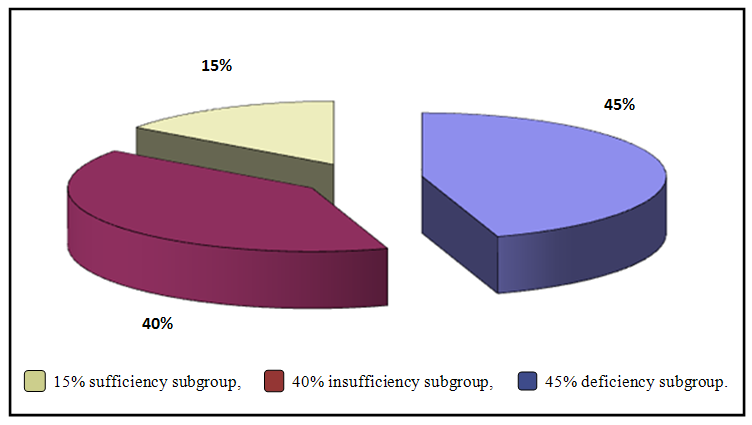 | Figure (1). Distribution of percentage of vitamin D subgroups among ulcerative colitis patients group |
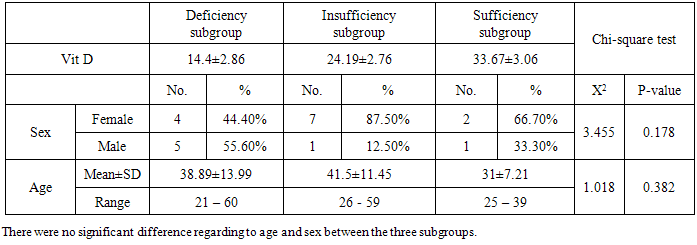
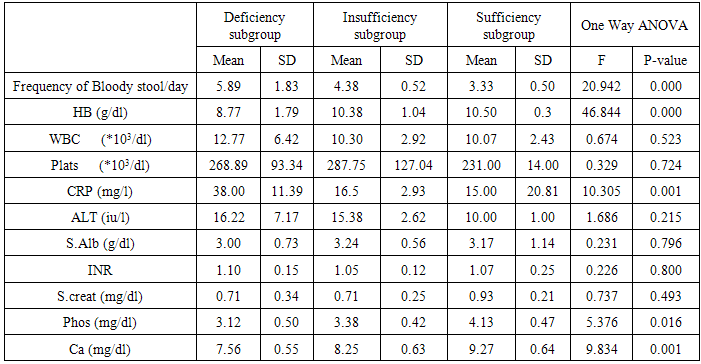
The results of table (7) showed: there were statistically highly significant difference between frequency of bloody stools / day, HB, CRP and ca and vitamin D subgroups (p= 0.000, 0.000, 0.001, 0.001 respectively). Also, there was statistically significant difference between serum phosphate and vitamin D subgroups (p=0.016), however, no significant difference with other studied parameters. Analysis of these findings support, the suggestion that, the greater the activity of ulcerative colitis, the greater the deficiency of vitamin D levels.Table (6). Comparison between vitamin D subgroups as regard to age and sex
 |
| |
|
Table (7). Comparison between vitamin D subgroups regarding the studied parameters
 |
| |
|
4. Discussion
The pathogenesis of inflammatory bowel disease (IBD) is thought to involve a complex interplay between genetic, environmental and microbial environments in the context of an inappropriate and abnormal activation of the mucosal immune system. Particularly, a dysregulated intestinal mucosal T-cell mediated immune response, specifically CD4 T helper type I (Th I) lymphocytes, leads to production of Th I-associated pro-inflammatory cytokines such as interferon-ɣ (IFN-ɣ) and tumor necrosis factor-alpha(TNF-α) [11]. There is increasing interest in the role of vitamin D in IBD, outside of its traditional role in metabolic bone disease. Novel insights into additional roles for vitamin D are being established and these include anti-inflammatory and immune modulating effects. Active vitamin D is known to exert its biological functions via the vitamin D receptor (VDR). Immune cells have been found to express VDR and possess the enzymes necessary to produce active vitamin D. This suggests vitamin D may have actions beyond endocrine activity [3]. The discovery of vitamin D receptor in most tissues and cells in the body has provided new insights into the function of this vitamin. There has been a dramatic increase in research examining the role of vitamin D and its receptor in many chronic illnesses, including cancers, cardiovascular disease, and autoimmune diseases such as multiple sclerosis and IBD [12].The present study was conducted to assess the vitamin D status (levels) in patients with ulcerative colitis in Egypt. The results obtained from this work were showed the mean serum values of 25(OH) vit. D in patients group(I) were lower in comparison to control group(II) (23.0±13.49ng/dl and 36.2±5.9 ng/dl) respectively, which was statistically highly significant (p<0.007), these results were in-agreement with El-Matary et al [9], who described lower 25(OH) vit. D concentration in a cross sectional study of children with newly diagnosed IBD compared to controls. Also, Dumitrescu et.al [8] found that, vitamin D levels were significantly lower in IBD patients compared to controls (24±10 ng/ml VS 31±13 ng/ml, p<0.05), however, IBD type did not influence this decrease, as no difference was found between crohn’s disease (CD) and ulcerative colitis(UC) patients. In parallel with our findings, Amiriani T. et al [1] reported that, the main serum levels of vitamin D in patients with ulcerative colitis was significantly lower as compared to the control group. The current study revealed that evidence of active ulcerative colitis was associated with lower vitamin D levels, there were inverse correlation between both frequency of bloody stools/day and C-reactive protein(CRP), which reflect activity of ulcerative colitis disease, with the vitamin D levels, which was statistically highly significant(p= 0,003 -0.000, respectively). Direct correlation between serum ca, phosphate, HB, and vitamin D levels, which were statistically significant, As, decreased these parameters were accompanied with decreased vitamin D levels. However, there were no significant correlation between, age, S. Albumin, WBCs, Platelets, INR, and S. Creatinin with vitamin D levels in UC patients. This study findings were in accordance with Blanck and Aberra [2], they concluded that, patients who were vitamin D deficient were more likely to have clinically active disease compared to patients with normal vitamin D levels, also, vitamin D appears to be an important factor in ulcerative colitis clinical disease severity in humans. The results of this work were inagreement with, viet et.al [20], as they considered, increased Erythrocyte sedimentation rate (ESR) is a sign of activity of IBD. They found that IBD patients with elevated ESR had significantly lower vitamin D levels than IBD patients with normal ESR levels, when compared to controls, patients with IBD and normal ESR level had no significant difference of vitamin D levels in comparison to controls, whereas patients with IBD and elevated ESR had significantly lowered vitamin D than controls. The findings of vitamin D deficiency in patients with IBD and elevated ESR suggest that inflammation is a risk factor for vitamin D deficiency in IBD, therefore, it may be prudent to closely monitor patients with IBD and elevated ESR for vitamin D deficiency. Contradictory to our study results, Dumitrescu et. al [8], who confirmed that, there were no statistically significant associations between vitamin D levels and smoking or medications status or with serum level of CRP or fibrinogen or ESR. Also, in disagreement with our study findings, Levin et.al [15], found that, no a correlation between vitamin D levels and disease activity in cross- sectional studies of IBD. The strongest evidence for relationship between vitamin D and VDR with IBD come from experimental mouse models. IL-10 knockout mice have been the prototypic IBD model, as these mice spontaneously develop enterocolitis at 5-8 weeks of age. Entrocolitis develops in these mice due to an uncontrolled immune response to microflora normally found in the gut [16]. A total of 30% of these mice went on to die from severe anemia and weight loss, particularly in mice that were found to be vitamin D deficient [18]. Cantorna, et. al [5], showed that vitamin D deficiency exacerbateenterocolitis symptoms in the IL10 KO mice. Furthermore VDR/IL10 double KO mice developed severe symptoms as early as 3 weeks and were all dead by 8 weeks. However, treatment with vitamin D led to amelioration, improved histologic scores and reduced mortality. This further supports the likely role of vitamin D in immune regulation and suggests a potential role as a therapeutic agent in IBD [4]. In the present study, ulcerative colitis patients (group I) were classified to 3 subgroups according to the level of vitamin D [8]: deficiency subgroup (9 patients) (45%), with main serum value of vitamin D(14±2.86 ng/ml) - Insufficiency subgroup (8 patients) (40%), with main serum value of vitamin D (24.19±2.76 ng/ml) and sufficiency subgroup (3 patients) (15%) with main serum value of vitamin D .(33.76±3.06 ng/ml). In comparison of 3 subgroups with studied clinical and laboratory items, showed, there were statistically significant difference regarding, frequency of bloody stools/day, HB, CRP, and Ca which had explained, the more severity of ulcerative colitis disease activity, the more deficiency of vitamin D levels, these results in accordance with Blanck and Aberra [2] who stated that, the proportion of patients having active UC disease increased as level of vitamin D decreased. The current study was revealed, the percentage of deficiency subgroup (45%), insufficiency subgroup (40%), sufficiency subgroups (15%), inulcerative colitis patients group, these findings, were in accordance with Mouli and Ananthakrishnan [17], who reported that, vitamin D deficiency is common in patients with inflammatory bowel diseases (16-95%), including those with recently diagnosed disease, evidence supports immunological role of vitamin D in IBD. Also, who concluded that, there is growing epidemiological evidence to suggest a role for vitamin D deficiency in the development of IBD and its influence on disease severity.
5. Conclusions
Vitamin D deficiency is common among Egyptian patients with ulcerative colitis, the increased degree of vitamin D deficiency, is associated with the increased degree of activity of ulcerative colitis disease, which may support suggestion of vitamin D status is a potential contributing factor underlying pathogenesis and disease activity of ulcerative colitis.
6. Recommendations
More understanding the potential role of vitamin D- deficiency as important contributing factor underlying the pathogenesis or a consequence of ulcerative colitis, the impact on its severity, and the effect of vitamin D supplementation on ulcerative colitis outcome, which may provide insight to possible therapeutic or preventive measures for ulcerative colitis patients, merits, multicentric, comprehensive studies on a large number of UC patients for long periods and on different stages of disease activity.
References
| [1] | Amiriani T, Barzanoni. S, Sedighy. S, et al. Vitamin D in ulcerative colitis, A cause or an Effect. Journal of clinical and Diagnostic Research (2012), 6, (6) 1011-13. |
| [2] | Blanck. S. and Aberra F. Vitamin D deficiency is associated with ulcerative colitis disease activity. Dig Dis Sci (2013) 58: 1698-1702. |
| [3] | Butts K and Beshardas K. Are we measuring vitamin D in inflammatory bowel disease(IBD) patients. Gut (2013); 62: 1123-1136. |
| [4] | Cantorna MT, Munsick C, Bemiss C, et al. 1, 25 Dihydroxycholecalciferol prevents and amelioration symptoms of experimental murine inflammatory bowel disease. J Nutr (2000); 130: 2648-52. |
| [5] | Cantorna MT, Zhu Y, Froicu M, et al. Vitamin D status, 1, 25- dihydroxyvitamin D3, and the immune system. Am J ClinNutr. (2004); 80: 1717S- 1720S. |
| [6] | Cosnes J, Rousseau GC, Seksik P et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroentrology (2011); 140: 1785-1794. |
| [7] | D’Haens GR, Panaccione R, Higgins PD, et al. The London Position Statement of the World Congress of Gastroentrology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose and how to predict response? Am J Gastroenterol (2011); 106:199-212. |
| [8] | Dumitrescu G, Mihai C, Dranga M, et al. Serum 25- hydroxyvitamin D concentration and inflammatory bowel disease characteristics in Romania World J Gastroenterology (2014) 20 (9): 2392-2396. |
| [9] | EL-Matary W, Sikora S, Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Dig Dis SCi (2011) 56: 825-829. |
| [10] | Froicu M, Weaver V, Wynn T, et al. A crucial role for the vitamin D receptor in experimental inflammatory bowel disease. MolEndocrinol. (2003); 17: 2386-2392. |
| [11] | Gibson PR.: Increased gut permeability in Crohn’s disease: Is TNF the link. Gut (2004); 53: 1724-1725. |
| [12] | Jahnsen J, Falch JA, Mowinckel P, et al. Vitamin D status , parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand J Gastroenterol. (2002); 37: 192-199. |
| [13] | Kornbluth A, and Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroentrol (2010); 105: 501-23. |
| [14] | Lappe J, Travers G, Davies K, et al. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am J ClinNutr (2007); 85: 1586-1591. |
| [15] | Levin AD, Wadhera V, Leach ST, et al. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci (2011); 56: 830-836. |
| [16] | Lim WC, Hanauer SB, Li YC. Mechanisms of disease: Vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. (2005); 2:308-315. |
| [17] | Mouli VP, and Ananthakrishnan AN. Vitamin D and inflammatory bowel diseases. Aliment.Pharmacol. Ther. (2014); 39 (2): 125-136. |
| [18] | Szodoray P, Nakken B, Gaal J, et al. The complex role of vitamin D in autoimmune diseases. Scand J Immunol. (2008); 68: 261-269. |
| [19] | Truelove and Witts. Disease activity in U. C Journal of Crohn’s and Colitis (2008); 2: 1-23. |
| [20] | Viet LE, Maranda L, Fong J, et al. The vitamin D status in inflammatory bowel disease. PLOS (2014); 9 (7): 1-7. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





