-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(4): 158-163
doi:10.5923/j.ajmms.20150504.03
Study of Urinary Neutrophil Gelatinase Associated Lipocalin-2(uNGAL) as a Marker in Renal Disease Activity with Systemic Lupus Erythematosis (Lupus Nephritis)
Eman M. I. Youssef1, Haneya A. A. Ali2, Nashwa El-Khouly3, 4
1¹Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2²Department of Microbiology & Immunology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3³Department of Internal Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
4Department of Internal Medicine, Faculty of Medicine, Taibah University, KSA
Correspondence to: Eman M. I. Youssef, ¹Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
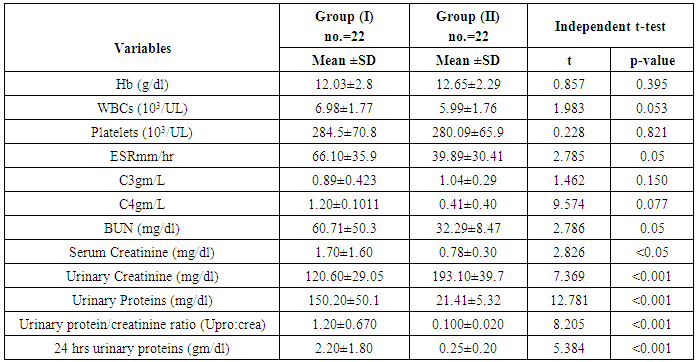
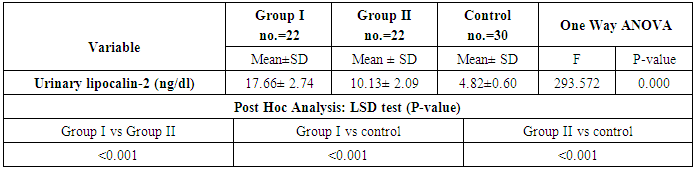
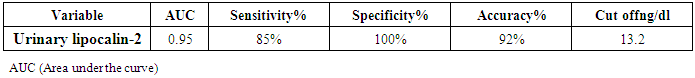
Background:Renal involvement in systemic lupus erythematosus (SLE) is a common manifestation and a strong predictor of poor outcome. Clinically significant renal involvement ranges from asymptomatic urinary findings to nephritic syndrome and renal failure. Several urinary chemokines and cytokines have been investigated in an attempt to complement and/or find an alternative to renal biopsy. Among these, lipocalin-2 is a promising one; therefore this study aims to assess the role of urinary lipocalin-2 as a biomarker in the diagnosis of lupus nephritis patients. Subjects and Methods: Measurement of urinary lipocalin-2 by ELISA in 44 patients with SLE were divided into two groups; twenty two patients with lupus nephritis (LN) as group (I), and twenty two SLE patients without LN as group (II), in addition to thirty healthy controls of matched age and sex as group (III). Results: Urinary lipocalin-2 levels were significantly higher in patients with lupus nephritis than in the SLE patients without nephritis or the control group. The diagnostic reliability of urinary lipocalin-2 to discriminate SLE patients with nephritis from those without nephritis, it showed a sensitivity of 85% and specificity of 100%, at cut off value 13.2ng/dl, with accuracy 92%. Conclusions: Urinary lipocalin-2 could be used as an early marker for kidney function deterioration in SLE.
Keywords: Systemic Lupus Erythematosus (SLE), Lupus nephritis (LN), Urinary lipocalin-2
Cite this paper: Eman M. I. Youssef, Haneya A. A. Ali, Nashwa El-Khouly, Study of Urinary Neutrophil Gelatinase Associated Lipocalin-2(uNGAL) as a Marker in Renal Disease Activity with Systemic Lupus Erythematosis (Lupus Nephritis), American Journal of Medicine and Medical Sciences, Vol. 5 No. 4, 2015, pp. 158-163. doi: 10.5923/j.ajmms.20150504.03.
Article Outline
1. Introduction
- Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease characterized by production of autoantibodies and formation of immune complex [1, 2], which mainly affects women in the childbearing period [3]. Multiple autoantibodies induce tissue damage either by binding directly to self-antigen or inducing inflammation following the tissue deposition of immune complex which occurs in many tissues including glomeruli, skin and lungs leads to manifestations of the disease [4, 5]. Kidney involvement is a major concern in SLE, affecting around 50% of patients and accounting for significant morbidity and mortality. Lupus glomerulonephritis (Lupus Nephritis) is the diagnosis applied to people with renal inflammation occurring in the SLE [6, 7]. In patients with severe lupus nephritis (WHO class IV) the incidence of end stage renal failure may reach up to10-26% [8]. LN can exist without visible symptoms, often no pain or swelling is noticed. Frequent urination at night might suggest the loss of protein in the body. If large amounts of protein are lost in the urine, nephrotic syndrome may occur. This may lead to symptoms like edema of the feet, ankles and legs. These are often the first noticeable signs of lupus nephritis [9, 10]. Thus a sensitive and a non-invasive biomarker for LN may be essential. The laboratory tools used to measure kidney damage such as proteinuria, haematuria, pyruia, presence of urinary casts, and serum creatinine levels are crude and relatively imprecise in their ability to indicate a flare in lupus nephritis, however renal biopsy remains the gold standard in diagnosing nephritis [11, 12]. An ideal biomarker for lupus nephritis should possess good correlation with renal activity as reflected by the degree of proteinuria and urine sediments, and specific to SLE for aiding the diagnosis of lupus nephritis. In addition, a useful biomarker should be easy to assay, simple to interpret, and readily available in most laboratories with a reasonable cost [13].Lipocalin-2 also known as Neutrophil Gelatinase- associated Lipocalin-2 (NGAL) and appears in three forms: as a 25 kDa monomer, a 46 kDa disulphide-linked homodimer, and a 135 kDa disulphide-linked heterodimer neutophil gelatinase-B, with its name neutrophil gelatinase-B associated lipocalin (NGAL). Lipocalin-2 was first described in human neutrophil granules as NGAL [14]. Lipocalin-2 is a protein that plays an important role in iron transport. The protein is produced in the immature neutrophil precursors in the bone marrow and stored in specific granules for subsequent release [15]. Lipocalin-2 is also expressed at a low level in other human tissues, including the kidney, prostate, epithelia of the respiratory and alimentary tracts. Under normal conditions lipocalin-2 promotes differentiation and structural organization of renal epithelial cells where lipocalin-2 was isolated from a ureteric bud cell line [16]. In renal injury, lipocalin-2 is highly accumulated in the human kidney cortical tubules, blood and urine, after nephrotoxic or ischemic injuries, thus lipocalin-2 might represent an early, sensitive, non-invasive biomarker for acute renal injury [17, 18].The objective of the present study was to evaluate the role of urinary lipocalin-2 in detecting the renal involvement in SLE patientsin order to detect an early, clinically accurate and available diagnostic tool for the diagnosis of lupus nephritis.
2. Subjects and Methods
2.1. Subjects
- The current study included 74 subjects aged between 22 to 47 years old. Forty four consecutive SLE patients were attending the outpatient clinic of Internal Medicine Department of Al-Zahara University Hospital, Cairo, Egypt from June 2014 to February 2015 and divided into 2 groups: Twenty two patients with LN as group (I), and twenty two SLE patients without LN as group (II). Thirtyhealthy subjects' sex and age matched to the patients served as control group (III). A written informed consent was obtained from all subjects enrolled in the study after explaining the study’s purpose, procedures, and possible benefits to them. All patients were diagnosed according to the American College of Rheumatology (ACR) diagnostic criteria. Lupus nephritis patients were defined by persistent proteinuria (<0.5 g/24 h), the presence of cellular casts, persistent hematuria or renal biopsy showing focal proliferative, diffuse proliferative or membranous glomerulonephritis. Patients with diabetes mellitus, malignanciesand those with a diagnosis of mixed connective tissue disease were excluded. Among the patients with LN, those undergoing hemodialys were excluded.
2.2. Methods
- All patients and controls were subjected to complete history taking, full clinical and routine laboratory assessments used to evaluate renal functionin the form of 24 hours urinary proteins to detect proteinurea, urinary protein and the urinary creatinine to calculate the urinary protein/creatinine ratio (Upro: crea) being a better and more accurate tool in detecting proteinurea, serum creatinine, blood urea nitrogen (BUN), serological markers for systemic lupus activity, including anti-double stranded deoxyribonucleic acid (anti-ds DNA), anti-nuclear antibodies (ANA), and autoimmune profile complement components C3 and C4 levels, complete blood picture (CBC) and erythrocytes sedimentation rate (ESR).
2.3. Detection of Human Lipocalin-2/NGAL by ELISA Method
- The RD191102200R Human Lipocalin-2/NGAL ELISA is a sandwich enzyme immunoassay for the quantitative measurement of human lipocalin-2 (BioVendor, Asheville, NC28806USA), according to the manufacturer’s instruction.
2.4. Statistical Analysis
- Data were collected, revised, coded and entered to the statistical package for social science (SPSS) version 20. Numerical data were expressed as mean ± standard deviation (SD).The comparison between means of the two groups was done by using Student's t-test and the comparison between the studied groups were done by using One Way Analysis of Variance (ANOVA) followed by post hoc analysis – least significant difference (LSD) test. Spearman correlation co-efficient test was used to rank variables versus each other positively or inversely. The receiver operating characteristic curve (ROC) was used to assess the best cut off point with its sensitivity, specificity and accuracy. The confidence interval was set to 95% and the margin of error accepted was adjusted to 5%. So, probability value (P-value) was considered insignificant at the level of more than 0.05, significant at the level of less than 0.05 and highly significant at the level of less than 0.01.
3. Results
- The results obtained are presented in tables (1-4) and figures (1-4). Detailed laboratory data of the patients with and without nephritis are shown in Table (1).SLE patients were 35 females and 9 males, the disease duration in all patients ranged from 1 to 10 years. Thirty healthy control subjects matched for age and sex with all patients, all controls had normal values for complete urine analysis. The ages of all participants ranged from 22-47 years with mean of 30.12±6.8 years. All patients had a positive ANA (100%); eleven patients had positive anti-dsDNA (50%) in each group (with and without nephritis).
|
|
|
|
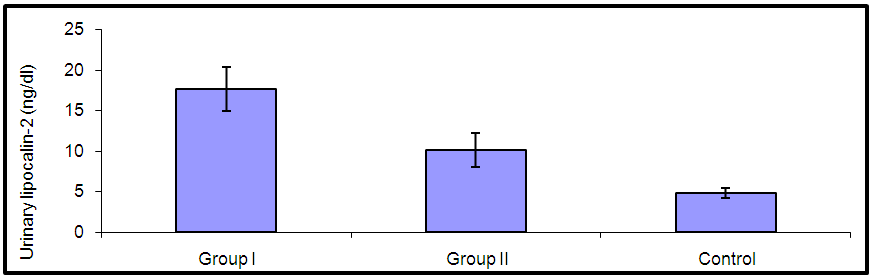 | Figure (1). Comparison of urinary lipocalin-2 among all studied groups |
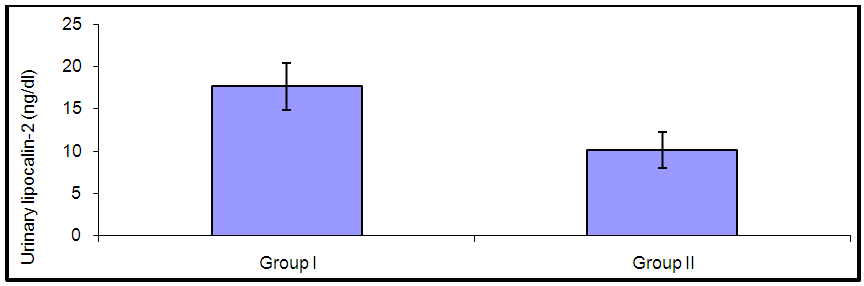 | Figure (2). Comparison of urinary lipocalin-2 between group I (SLE with nephritis) and group II (SLE without nephritis) |
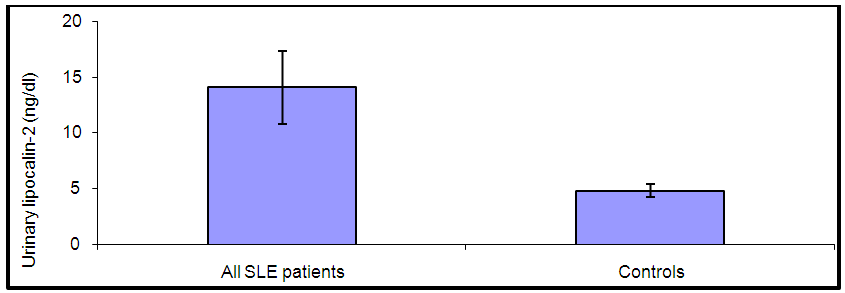 | Figure (3). Comparison between levels of urinary lipocalin-2 in all SLE patients and controls |
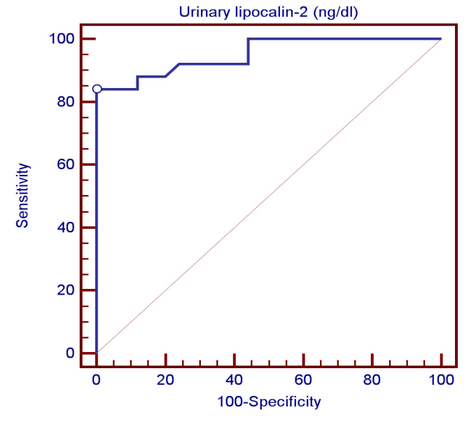 | Figure (4). Receiver operating characteristic (ROC) curve analysis showing urinary lipocalin-2 for detection of lupus nephritis |
4. Discussion
- By studying lipocalin-2 in different groups, the urinary lipocalin-2 had the highest mean levels in LN patients (group I) compared to other groups (p< 0.001), also significant difference between SLE patients without nephritis (group II) and controls (p< 0.001) (Table 2 & Figure 1). Theurinary lipocalin-2 were significantly higher mean levels in all SLE patients compared to healthy controls (p< 0.001) (Figure 3), also significant difference between LN patients when compared to SLE patients without nephritis (p< 0.001) (Figure 2). These findings come in consistent with previous work done by Farzaneh et al. [19] found that the study was conducted on fifty two lupus patients. Urinary lipocalin-2 levels in LN patients were significantly higher than those in non-LN patients (P-value= 0.03). Also, our results were in accordance with the study done by Hammad et al. [20] who found that the study was conducted on33 children with active SLE (22 with and 11 without LN) and compared to 15 matched controls. Levels of urinary NGAL were higher in patients with LN than those without LN (P<0.001). Urinary NGAL is a sensitive marker of proliferative nephritis in juvenile SLE. Also, Suzuki et al. [21] found results that match with our current one where they reported that urinary lipocalin-2 levels were significantly higher in all SLE patients than healthy controls. The authors explained these findings that the elevation of urinary lipocalin-2 might be a reflection of renal injury/inflammation. Similarly, Pitashny et al. [22] who found significantly higher levels of urinary lipocalin-2 in SLE individuals in the presence of nephritis than in its absence compared with the values in normal controls. The elevated levels of urinary lipocalin-2 in SLE patients with active nephritis are explained that the urinary lipocalin-2 levels are produced mainly by the injured tubular cells, in direct proportion to the degree and severity of the disease. Yet in contrast to our results, the study done by Bu [23], they found no significant difference in urinary lipocalin-2 seen between SLE patients without nephritis and the control group. The correlation of laboratory parameters used in our study showed a significant positive correlation of urinary lipocalin-2 and each of the urinary protein /creatinine ratio (Upro: crea), BUN, serum creatinine. This coincides with Pitashny et al. [22] who found similar results to our current study in which there was a positive correlation of urinary lipocalin-2 levels and the urinary protein/ creatinine ratio (Upro/cre). These results also agree with the study reported by Bolignano et al. [24], who also showed that urinary lipocalin concentrations correlated with renal function and a strong correlation existed between urinary lipocalin and the presence of proteinuria. While the most likely source of urinary lipocalin in acute kidney injury is the distal tubules, the evidence of a strict correlation between urinary lipocalin and the degree of proteinuria may result from increased glomerular protein loss and disturbed reabsorption in the proximal nephron segment in addition to increased intrarenal production, so in response to large amounts of filtered protein due to a glomerular capillary leak, it is possible that urinary lipocalin is released from the renal tubular epithelium. Similar results were also reported by Brunner et al. [25], who found that urinary lipocalin-2 level is associated with renal disease activity and with the urinary protein/urinary creatinine ratio (Upro: crea) as being the representative of the renal involvement. Also, Koura et al. [26], who pointed out that the lipocalin-2levels, were strongly correlated with renal disease activity but not with extra renal disease activity score.The positive correlation that was found in our study between lipocalin-2 and urinary protein /creatinine ratio (Upro: crea) suggests that lipocalin-2 may be a new sensitive biomarker of lupus nephritis with its levels being increased in patients with active renal disease. Among previous reports, Chi [27], who found the same results where urinary lipocalin-2 levels correlated with urine protein/ creatinine ratios and were associated with renal SLE disease activity, but not with global damage or extrarenal disease activity.Finally, the diagnostic reliability of lipocalin-2 in the present work showed a good performance of urinary lipocalin-2 to discriminate SLE patients with nephritis from those without nephritis with area under the curve (AUC) value 0.95 at a cut-off value of 13.2 ng/dl (sensitivity 85% and specificity 100%) which supports the above results that lipocalin-2 can be used with a lot of trust to early diagnose lupus nephritis. In agreement with a recent study done by Torres-Salido et al. [28] who reported that the urinary neutrophil gelatinase-associated lipocalin (NGAL) level was found to be the best candidate to predict proteinuric flares with an 87% sensitivity and 62% specificity for ratios >14.56 and complete response with a 61% sensitivity and 78% specificity for ratios >26.54 and a reliable marker of disease activity in patients with SLE and could be used as an indicator of response to therapy. Meanwhile Pitashny et al. [22] found AUC 0.71 at a cutoff value of >28 ng/mg, the sensitivity of urinary lipocalin-2 levels for the diagnosis of LN was 50%, with a specificity of 91%.Similar results were also reported by Hammad et al. [20]. Urinary NGAL was significantly predictive of class III and IV LN (P=0.005) with 91% sensitivity and 70% specificity to levels ≥ 10.07 ng/mg and was a sensitive marker of proliferative nephritis in juvenile SLE. The area under the curve (ROC curve) of urinary lipocalin-2 was 0.94 as found by Brunner et al. [25] in an adult population and showed a sensitivity of 50%, a specificity of 91%, in a pediatric cohort, urinary lipocalin-2 values had a sensitivity of 90% and a specificity of 100% for identifying patients with lupus nephritis. Therefore urinary lipocalin-2 levels showed significantly higher levels in lupus nephritis patients compared to other SLE patients and controls being superior to creatinine regarding detecting the renal disease activity in the SLE patients.
5. Conclusions
- An important clinical conclusion is that adding measurement of urinary lipocalin-2 to the routine follow-up of LN patients may result in earlier diagnosticmarker for kidney function deterioration in SLE, provide additional clinically relevant information about disease activity to that given by the established marker and therefore less delay in institution of appropriate treatment. Thus, our results are important as they suggest a novel approach to the clinical management of lupus patients. Possibly, determination of urinary lipocalin-2 can be helpful in the evaluation of lupus nephritis in general. Our study have a limitation that we assessed the levels of urinary lipocalin-2 in a limited number of patients and should be higher than the number included in this study. Greater number of patients is recommended to gain greater insight into potential usefulness of urinary lipocalin-2 in patients with SLE especially LN patients. Further studies should be done on the urinary lipocalin-2 to support its utility in the diagnosis of renal disease activity and severity in SLE patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML