-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(3): 135-139
doi:10.5923/j.ajmms.20150503.05
Role of Prolactin in Iraqi Obese Women with Polycystic Ovary Syndrome
Jasmine S. Hasan1, Alia H. Ali1, Muhammad-Baqir M-R. Fakhrildin2
1College of science for Women, Baghdad University, Baghdad, Iraq
2High Institute for Infertility Diagnosis and Assisted Reproductive Technologies, AL-Nahrain University, Baghdad, Iraq
Correspondence to: Jasmine S. Hasan, College of science for Women, Baghdad University, Baghdad, Iraq.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Prolactin is a polypeptide hormone which produces by the pituitary gland during pregnancy and lactation. In some cases, it is slightly elevated in polycystic ovary syndrome (PCOS). This study was aimed to find the relationship between prolactin (PRL) level and BMI in women with polycystic ovary syndrome. The present study was carried out on (51) PCOS women and (10) healthy women as controls at High Institute for Infertility Diagnosis and Assisted Reproductive Technologies/AL-Nahrain University, from February to August, 2014. Prolactin and OGTT were performed for all women. Descriptive data including age and BMI were recorded for all subjects. Significant increment (P<0.05) in the BMI value was observed for PCOS subgroup with insulin resistance and for PCOS women with age (25.63±0.676 years). Significant increment (P<0.05) of serum PRL level was observed for obese PCOS women with insulin resistance as compared with controls. Also, highly significant increment (P<0.05) of PRL level was assessed in PCOS age group (A) with mean age (18.91±0.285) and (B) with mean age (25.63±0.676) as compared with controls. OGTT observed in obese women and in PCOS women with age mean (25.63±0.676) were significantly increased (P<0.05) as compared with controls. In conclusion, PRL level was associated with obesity and insulin resistance in PCOS patients.
Keywords: Prolactin, Obese Women, Polycystic Ovary Syndrome
Cite this paper: Jasmine S. Hasan, Alia H. Ali, Muhammad-Baqir M-R. Fakhrildin, Role of Prolactin in Iraqi Obese Women with Polycystic Ovary Syndrome, American Journal of Medicine and Medical Sciences, Vol. 5 No. 3, 2015, pp. 135-139. doi: 10.5923/j.ajmms.20150503.05.
1. Introduction
- Prolactin (PRL) is a polypeptide hormone produced by the pituitary gland and plays an important role in the differentiation of normal mammary epithelium. It's contained 200 amino acids and prepares the physiology for lactation and maternal care in women [1]. The elevation in PRL levels is normal during pregnancy and breastfeeding. Furthermore, PRL can abnormally increase because of a disease or the use of certain medications [2]. For unclear reasons, some of the PCOS women may have a slightly increment in prolactin levels [3].Polycystic ovary syndrome (PCOS) is a common endocrine disorder in women of reproductive age, which affects 5-10% of women [4]. About 50% of PCOS patients are obese or they have a greater risk of overweight, obesity, and central obesity [5]. Obesity is a common finding in PCOS and aggravates many of its reproductive and metabolic features. The correlation between PCOS and obesity is complex and not well understood, which involves the interaction of genetic and environmental factors [6]. Women with high PCOS have 76.5% prevalence of insulin resistance and upper body obesity which in turn aggravates insulin resistance and hyperandrogenism of women with PCOS and modulate β–cell function [7]. PCOS case similar to hyperprolactinemic are both common causes of secondary amenorrhea in women. The correlation between PCOS and hyperprolactinemia so far has been reported still with argumentative results. It appears that PCOS is very dominant with hyperprolactinemia, though there are variant reasons of altered regulation of gonadotropin secretion and suggests that these conditions have independent origins [3]. Therefore, the aim of this study is to find the relationship between prolactin level and obesity in PCOS women.
2. Materials and Methods
- This study was carried out at the laboratory of Hormonal and Biochemical assays of High Institute for Infertility Diagnosis and Assisted Reproductive Technologies / AL-Nahrain University from February to August, 2014. From a total of 61 women with age range 17-49 were studied, 51 women were suffering from PCOS, and 10 healthy women were considered as controls to compare with PCOS's women. Blood samples (2.5 mL) were collected from each fasting woman in the morning, and 75 gm of glucose loaded in 400-500 mL of water and given to the subjects. The collected blood was transferred to gel and clot activator tube and incubated for 10 minute in the incubator at 37℃ to coagulate, and serum separated by centrifuge and freezing in -4℃. Prolactin assay was performed to all subjects using mini Vidas kit (Biomerieux, France). The normal value of prolactin range 5-35 ng/mL. OGTT test was performed after 30, 60, and 90 minute after glucose loading. GTT was performed using glucose MR kit of Linear (Spain). The normal value of OGTT is <140 mg/dl, OGTT between 140-199mg/dl refer to impaired glucose tolerance, and its considered diabetic if OGTT ≥ 200 mg/dl [8]. Body mass index (BMI) was measured for all subjects by dividing weight in kilogram on height in meter square. BMI value indicates for body weight, subjects with BMI 18.5-24.9 Kg/m2considered normal weight, with BMI 25-29.9 Kg/m2 considered overweight, and with BMI ≥ 30 Kg/m2considered obese. The duration of infertility recorded for all infertile PCOS patients. Statistical analysis was performed to all data using Statistical Package for Social Science (SPSS); version 20. The differences among more than two groups were assessed by using ANOVA table- Duncan test with means and standard error of means. The confidence level has been chosen as 95% and P-value <0.05 was considered as significant. The Pearson correlation coefficient was estimated between PRL and BMI. The correlation value was considered statistically significant at the level (P<0.05).
3. Results
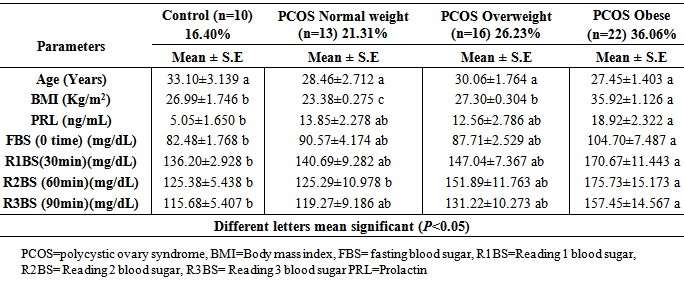
- Table (1) demonstrated a comparison among three groups according to OGTT, including; PCOS with insulin resistance (IR), PCOS without insulin resistance (NIR) and control group. No significant differences (P>0.05) were observed in the age among these three groups. There was significant increment (P<0.05) for BMI of PCOS-IR subgroup than both PCOS-NIR and control group. Also, there was no significant difference (P>0.05) in BMI between PCOS-NIR subgroup and control group. The level of serum PRL was significantly increased (P<0.05) in PCOS-IR when compared with healthy controls women. While non-significant differences (P>0.05) were observed between PCOS-NIR and both PCOS-IR and controls. According to GTT, PCOS women were divided into PCOS-IR and PCOS-NIR as shown in the same table, significant increment (P<0.05) was recorded in the level of fasting blood glucose for PCOS-IR than control group, but not significant (P>0.05) with PCOS-NIR women. Moreover significant elevation (P<0.05) in BS levels were recorded of reading one (R1BS after 30 minute), reading two (R2BS after 60 minute), and reading three (R3BS after 90 minute) in PCOS-IR than both PCOS-NIR and controls.
|
|
|
4. Discussion
- In present study, 52.45% of females with PCOS had IR according to higher OGTT (OGTT>140 mg/dl), and 31.15% of females with PCOS had not IR according to lower OGTT (OGTT<140 mg/dl) (Table 1). This result coincided with Legro et al. [9] and Recha et al. [10]. As well, they were obese or overweight which agrees with Hoeger, [11] and Gomathi et al. [12]. Additionally, when PCOS women divided into subgroups according to BMI values (Table 2), higher prevalence of PCOS women (36.06%) of females were observed obese, about 26.23% of females with PCOS were overweight, and about 21.31% of subjects with PCOS were normal weight that means PCOS case frequently higher in overweight and obese women, and the obesity play an important role in pathophysiology of this syndrome [13]. Several studies suggested that obese PCOS women had higher resistin level. The serum resistin level may increase in overweight and obese women in comparison with normal-weight women, irrespective of having PCOS [14]. In other study done by Seow et al. [15] concluded that resistin mRNA levels were two-fold higher in adipocytes from women with PCOS than in those from normal controls. In this study, PCOS women with IR had higher levels of OGTT in comparison with PCOS NIR (non-insulin resistance) and control group; obese PCOS subgroup; and according to age group, PCOS patient subgroup (B) (age mean 25.63 years) had higher OGTT with BMI >30 kg/m2. This situation is may due to the relation of resistin with obesity and insulin resistance which was agreement with Steppan et al. which they mentioned that resistin hormone leading to insulin resistance, while serum resistin level increase in the presence of obesity in mice, an anti-diabetic agent rosiglitazone decreased levels and that administration of recombinant resistin to mice caused impairment in glucose tolerance and insulin effect [16]. Also, Rekha et al. [10], concluded that IR was common in PCOS women and was practically more common in obese PCOS subgroup, and this conclusion in line with the present study. Thus, no significant differences in OGTT were observed between PCOS subgroup NIR and control group (Table 1), that mean not all PCOS women has IR, this result coincided with [17]. The higher significant prolactin level in obese PCOS may correlated to BMI according to many studies which observed higher prolactin levels in obese women [18], which supported that PRL level associated positively with obesity. Rosenfield was concluded that women with PCOS have a disruption of the neuroendocrine mechanisms including; a deficiency of hypothalamic dopamine regulating both gonadotropin- realizing hormone (GnRH) and prolactin release [19]. In a study done by Shibli-Rahhal and Schlechte [20] were concluded that the association between prolactin, weight gain and obesity suggests that prolactin may play a role in the modulation of body weight and composition, and they added it remains unclear whether weight gain is associated with hyperprolactinemia due to stimulation of lipogenesis or due to disruption of central nervous system (CNS) dopaminergic tone [20]. Also, Hernandz et al., [21] concluded that a hypothalamic deficiency of dopamine (DA) could explain the mild elevation in PRL level frequently present in women with PCOS, which is further supported by the finding of a low DA hypothalamic tone with increased prolactin bioactivity in obese, hyperinsulinemic women with PCOS which was agrees with this study [21]. Another study was done by Tuzcu et al. [22] on 60 hyperprolactinemic women and 12 healthy as control, and they found basal insulin level of hyperprolactinemic patients were higher than the control group, and they concluded that hyperprolactinemic patients were more insulin resistant than control subjects this conclusion agrees with the present study which illustrated higher glucose levels (OGTT>140mg/dl) (IR marker) were observed in obese and overweight patients and those patients had higher mild levels of prolactin [22]. Recently, Tuzcu et al. [22] demonstrated that prolactin induces glucose intolerance, hyperinsulinemia and insulin resistance in several animal species, and they concluded that hyperprolactinemia in women was associated with insulin resistance state [23].
5. Conclusions
- Higher prolactin level was associated with multiple disorders. It has a relation with obesity and insulin resistance in cases of PCOS. Moreover, prolactin plays a role in obesity condition for PCOS women. In spite of elevation level of serum prolactin in hyperinsulinemic and obese PCOS women when compared with healthy fertile women but still prolactin level within normal range. Further biochemical and molecular studies are recommended to investigate the correlation between appetite and level of serum prolactin in healthy and PCOS women.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML