-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(3): 126-129
doi:10.5923/j.ajmms.20150503.03
Study of VEGF-A Gene and NOS3 Polymorphism in the Patients with Nasopharyngeal Angiofibroma
Abdurakhmanov Otabek Bakhtiyarovich
Senior Researcher of Department of ENT, Tashkent Institute of Postgraduate Medical Education, Republic of Uzbekistan
Correspondence to: Abdurakhmanov Otabek Bakhtiyarovich, Senior Researcher of Department of ENT, Tashkent Institute of Postgraduate Medical Education, Republic of Uzbekistan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
There were studies of the incidence of the most characteristic polymorphic markers of vascular endothelial growth factor VEGF-A: C963T, G405C, -1154A and nitrooxigenase NOS3 – T786C and T894G in the patients with nasopharyngeal angiofibromas. Identified VEGF-A gene polymorphism suggests that single nucleotide substitutions in the promoter region of genes are unique and have a definite impact on features of functioning proteins and gene expression.There are presented rationality of the combined analysis of some genetic markers –G405C VEGF-A gene and T894G, T786C of NOS3 gene for increasing of efficacy of early diagnosis and formation of risk groups of peoples, inclined to development of the angiofibroma.
Keywords: VEGF-A, NOS, Nasopharyngeal angiofibroma, Gene polymorphism, Gene markers, Prevalence
Cite this paper: Abdurakhmanov Otabek Bakhtiyarovich, Study of VEGF-A Gene and NOS3 Polymorphism in the Patients with Nasopharyngeal Angiofibroma, American Journal of Medicine and Medical Sciences, Vol. 5 No. 3, 2015, pp. 126-129. doi: 10.5923/j.ajmms.20150503.03.
1. Introduction
- The vascular endothelial growth factor A (VEGF-A) is a member of the family of structurally closed proteins, which together with receptors (VEGFR) play a crucial role in development and regulation of activity both of the blood and lymphatic vessels. VEGF-A effects on the development of new blood vessels (angiogenesis) and on the survival of the immature blood vessels (vascular support), linking with two closed structurally membrane tyrosine kinase receptors (VEGFR-1 and VEGFR-2) and activating them [5]. Besides, the data of last years testify that VEGF-A is not only the main stimulator of angiogenesis, but also a lymphagenous factor [3, 4].Despite the fact that the identity of the human genome is extremely high level of gene sequences differences between the two individuals is about 0.1% [2]. The point mutations, that is, replacements of single nucleotides (SNP-single-nucleotidepolymorphism), appeared to be the most frequent reason of distinctions in the structure of genes. There has been revealed two classes of high-affined VEGF-А-linking sites on the cells and it is suggested that such sites are required for monocytes in VEGF-dependent chemo taxis. It is shown, that similar low molecular (120-130 kDa) receptors exist on the cells of a tumor and link VEGF-А165, but not VEGF-А121. Thus, the special type of a tumor and endothelial cells express low affined proteins which selectively link coded sequences [1].The increased contents of VEGF, previously shown, may be considered under these conditions as compensatory reactions directed to the restoration of the vascular density and reduction of the arterial pressure. Development of the angiofibromas is characterized by endothelial dysfunction due to neutralization of NO reactive oxygen forms forming as a result of stimulation of type I angiotensin II receptors, mitochondrial dysfunction, nuclear factor activation, cV, inflammatory induction and disturbance of the sensitivity to insulin [7]. Endothelial dysfunction is associated with increase in VEGF concentration that provides compensatory growth of the synthesis of NO and prostacycline, inducing vasodilatation [8].The purpose of the present study was to evaluate the prevalence of the most typical polymorphic markers of VEGF-A factor: C963T, G405C,-1154, and nitroxisynthetase NOS3 – T786C and T894G in the patients with nasopharyngeal angiofibroma.
2. Materials and Methods
- The method of pyrosequencing was used to define of the genetic markers. The technique of detection of genetic polymorphisms of VEGF-A genes and NOS3 included the following stages:1. DNA isolation from the clinical material. Standard methods were used with the set of reagents “DNA-sorb-B” manufactured by FGUN "Central Scientific Research Institute of Epidemiology" of Rospotrebnadzor. 2. PCR-amplification of a fragment containing polymorphic genetic locus. At amplification of the DNA fragment one of the pair of primers was linked at the 5’-end with biotin; the DNA chain, which will serve as a matrix for pyrosequencing, is amplified with biotinilated primer. 3. Preparation of the samples of PCR-product. This procedure included amplicon incubation with particles of sefarose, covered with streptavidin, amplicon denaturation and series of consecutive washings resulting in one-chained PCR- product, fixing on the particles of sefarose. 4. Immobilization of PCR-product on the solid surface and annealing of sequencing primer in the area of analyzing genetic locus. These processes resulted in duplex between DNA-matrix and sequencing primer, necessary for performance of reaction of pyrosequencing synthesis. 5. Sequencing of PCR-product – performance of the reaction of pyrosequencing and analysis of the results obtained.For evaluation of reaction of pyrosequencing we used systems of the genetic analysis PyroMark Q24. As the object of research was to characterized polymorphic locuses in the human genome, and position of the single-nucleotide polymorphism known there was used opportunity for automatic processing of the result with the software of the devices used. On the basis of relative height of peaks on the pyrogram homo- or heterozygous state was determined by polymorphic locus.
3. Results
- The results of VEGF-A genotyping in patients with nasopharyngeal angiofibroma are presented in table 1. The analysis of the data has shown that the patients, at the analysis of a genetic marker С963T, have a genotype СС, whereas the given genotype was not found out in the practically healthy people. At genotyping of a marker G405С of VEGF-A gene in group of the patients the increase in frequency of prevalence of a genotype CC (40,0 %) was noted, in comparison with control group (15,0 %). In case of research of G-1154A polymorphism the decrease (10,0 %) in prevalence of a genotype AAi, increase in quantity of detection of a genotype GG (50,0 %) in group of the patients with angiofibroma was observed, in comparison with the control (30,0 % and 10,0 %, respectively).
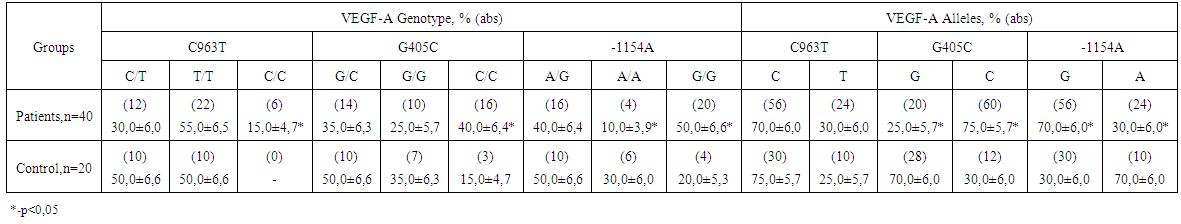 | Table 1. Distribution of the frequencies of VEGF-A gene genotypes in the patients with nasopharyngeal angiofibroma |
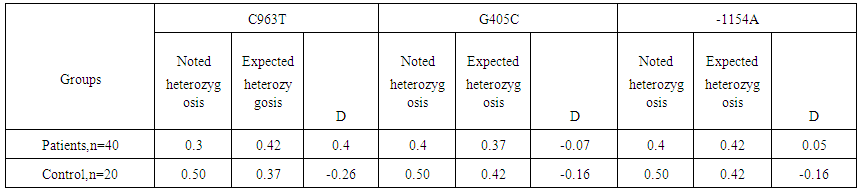 | Table 2. Differences between expected and noted frequencies of heterozygosis of gene VEGF-A genetic markers |
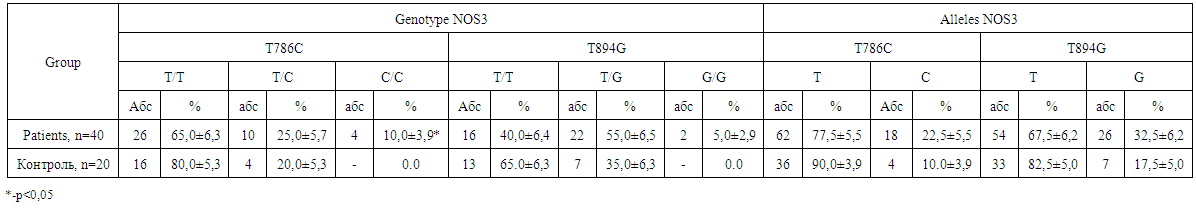 | Table 3. Distribution of the frequencies of NOS3 gene genotypes in the patients with nasopharyngeal angiofibroma |
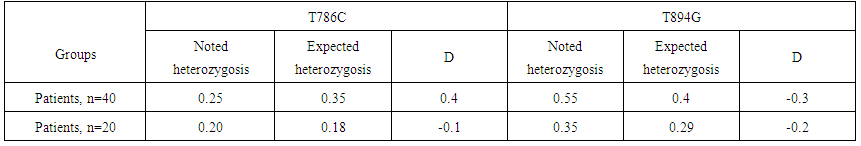 | Table 4. Differences between expected and observed frequencies of heterozygosis of genetic markers of gene NOS3 |
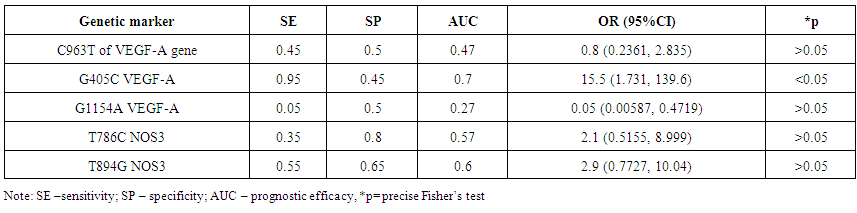 | Table 5. Results of identification of prognostic efficacy of studied genetic markers of genes VEGF-A and NOS3 |
4. Discussion
- Expression of VEGF is stimulated by set of the proangiogenic factors, including epidermal growth factor, main fibroblast growth factor, thrombocytary growth factor, interleukin 1β, and factors of external environment, such as рН, pressure and concentration of oxygen. The similar influence consists in mediated through VEGF stimulation of important for angiogenesis and lymphangiogenesis factors, including antiapoptotic proteins, molecules of cellular adhesion and metalloproteinase. However, the fact is conclusive, that production of VEGF in reply to standard stimuli varies between the people, and in a population there are met stable low-producing and high-producing phenotypes, at the constant structure of synthesized protein [6].The researches performed for revealing of the frequency of polymorphism prevalence of the VEGF-A gene allowed to establish, that in the patients with nasopharyngeal angiofibroma there was determined genotype CC of the genetic marker С963T, increase of CC genotype frequency up to 40,0 % at the analysis of a marker G405С, decrease of frequency of genotype AA up to 10,0 %, and increase of frequency of genotype GG up to 50,0 % at the analysis of a marker G-1154A. In the investigated groups of the patients and control at the analysis of genetic marker С963T there was observed distribution of frequencies of genotypes of this polymorphism without deviation from BHW, i.e., it was corresponded to expected (χ2=0.1; Р=2.2).
5. Conclusions
- 1. Revealed polymorphism of gene VEGF-A allows to assume, that single-nucleotide replacements in the promoter region are individualized and have special effect on the features of the protein functioning and on the gene expression. 2. In the patients with nasopharyngeal angiofibroma there was observed genotype CC of genetic marker С963T, increase in frequency of CC genotype to 40,0 % at the analysis of marker G405С, decrease in frequency of AA genotype up to 10,0 % and increase in frequency of GG genotype to 50,0 % at the analysis marker G-1154A.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML