-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(3): 121-125
doi:10.5923/j.ajmms.20150503.02
Molecular Study of Insulin Resistance and Polycystic Ovary Syndrome
Mohammad Ibraheem Mezaal, Mohammed Ibrahim Nader, Ismail Hussein Aziz
Ministry of Higher Education and Scientific Research, University of Baghdad, Genetic Engineering and Biotechnology Institute for Postgraduate Studies, Baghdad, Iraq
Correspondence to: Mohammad Ibraheem Mezaal, Ministry of Higher Education and Scientific Research, University of Baghdad, Genetic Engineering and Biotechnology Institute for Postgraduate Studies, Baghdad, Iraq.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This study explores on the relationship between the polycystic ovary syndrome (PCOS) and insulin resistance which caused by variation in the 5-prime flanking region of the insulin gene, a which regulates transcription of the gene. The study includes 50 Iraqi women with polycystic ovary syndrome (PCOS) and 50 healthy women, Blood samples were collected from the Medical City hospital, Kamal al-Samarrai Hospital and private medical laboratories (in Baghdad), during the period from November, 2011 to May, 2012. The age of infertile and fertile women was ranged from 16 to 45 years. The molecular study was focused on the 42% of PCOS women with insulin resistance. By sequencing for 50 samples, one separate segment (promoter) containing nucleotide 360 of insulin gene was amplified by using specific primer. The result of sequencing refers to a number of mutations found in women with PCOS (substitution = 69.86%, deletion =10.95% and insertion =19.17%). Also compound mutations have been detected among most of PCOS with insulin resistance.
Keywords: Polycystic ovary syndrome, Variable number of tandem repeat, Mutations, Sequencing
Cite this paper: Mohammad Ibraheem Mezaal, Mohammed Ibrahim Nader, Ismail Hussein Aziz, Molecular Study of Insulin Resistance and Polycystic Ovary Syndrome, American Journal of Medicine and Medical Sciences, Vol. 5 No. 3, 2015, pp. 121-125. doi: 10.5923/j.ajmms.20150503.02.
1. Introduction
- Polycystic ovary syndrome (PCOS) is one of the most common female endocrine disorders, but there is strong evidence that it can to a large degree be classified as a genetic disease [1]. PCOS produces symptoms in approximately 5% to 10% of women of reproductive age. It is thought to be one of the leading causes of female subfertility and the most frequent endocrine problem in women of reproductive age [2].The principal features are an ovulation, resulting in irregular menstruation, amenorrhea, ovulation-related infertility, excessive amounts or effects of androgenic hormones, hirsutism, and insulin resistance, often associated with obesity, Type 2 diabetes, and high cholesterol levels . Not all women with PCOS have polycystic ovaries (PCO), nor do all women with ovarian cysts have PCOS; although a pelvic ultrasound is a major diagnostic tool, it is not the only one. The diagnosis is straightforward using the Rotterdam criteria [3]. Women with PCOS are more insulin resistant, and up to 40% of these patients have impaired glucose tolerance. They are at an increased (3–7 times) risk of developing early-onset type 2 diabetes, the risk being greater in obese PCOS patients and those with a family history of type 2 diabetes. Insulin plays both direct and indirect roles in the pathogenesis of androgen excess in PCOS. Although women with PCOS have peripheral insulin resistance, ovarian steroidogenesis appears to be hypersensitive to insulin [4].In addition, insulin can stimulate human theca cell proliferation, and can also enhance ovarian growth and follicular cyst formation in rats [5]. A highly polymorphic stretch of DNA lying 360 bp upstream of the initiation of transcription of the INS gene which is located in an promoter region. Any mutations in this region may be effect on insulin gene (INS) expressions. Genotype of the insulin promoter were different among various population groups. Several studies and research focused on the genetic variations of genes that have an impact on causing Polycystic Ovary Syndrome (PCOS) such as insulin promoter and other region located in same gene (Insulin) [6].The aim of this study is to detect the relationship between PCOS and insulin resistance caused by mutations in promoter of insulin gene (non coding region) and hormonal disturbance with other parameters, to achieve this aim the following steps were done:1. Identification of some genetic mutations in the promoter of insulin gene that have a role regulating insulin action.2. Determination the presence of relationship between PCOS and insulin resistance.
2. Materials and Methods
- A. Subjects This study was carried out through period between “1 August 2011 to 30 of March 2012” in Institute of Genetic Engineering & Biotechnology for post Graduate Studies at University of Baghdad. Two study groups have been investigated:1. Patients group (PCOS group)This study has included fifty infertile Iraqi women with PCOS. Patients were selected from the Medical City Hospital and Kamal Al-Samarrai Hospital in Baghdad.2. Healthy control group (fertile)Healthy control group consists of fifty healthy fertile women of different ages (16-45 years). venous blood samples (5 ml) Have been collected from each women of both PCOS and healthy control.B. Genomic DNA isolationTotal genomic DNA isolated from the whole and frozen blood collected in EDTA anticoagulant tubes for molecular studies was applied using genomic DNA purification kits (Bioneer) /Korea. The isolation of DNA was based on five steps process using salting out methods [7].C. Agarose Gel ElectrophoresisAfter genomic DNA extraction, agarose gel electrophoresis was adopted to confirm the presence and integrity of the extracted DNA [7].The reagents of Gel Electrophoresis: ● Agarose.● 1 X TBE Buffer.● Bromophenol Blue in 1 % glycerol (loading buffer).● Ethidium Bromide. ● DNA Ladder Marker (100 bp).D. Insulin gene (INS) mutations detection The insulin (INS) gene locates at chromosome 11p15.5 and is one of established susceptibility locus to type II diabetes. In the 5-prime flanking region of the INS gene, a non coding region that regulates transcription of the gene. therefore specific primers was designed to amplification (INS-promoter) region in this study (PCR). The selection of these regions is based on their among most common insulin resistance that leads to PCOS [8].PCR process was performed using specific primer was designed for (INS-promoter) region by using (another studies, NCBI and Primer 3 programe). The sequences of these primers were listed in (table 1).
|
| |||||||||||||||||||||||||||||
3. Results and Discussion
- A. Age distribution of the samples:The age of all PCOS women was ranged less than 25 to more than 35 years. Table (3-1) revealed that 42% of the study PCOS women were between (25 -35) years, followed by 30% of patients whose age ranged between (>25) years and 28% of patients whose age (<35) years of total PCOS patients. This may leads to conclude that group 2 and group 3 constituted the greatest number groups in the present study. The results agreed with other studies that reported affecting 4% to 12% of women of reproductive age. [10].It is believed that the reason for this is due to genetic differences and geographic locations in addition to the environmental conditions and surrounded by physical and chemical effects.
| ||||||||||||||||||||||||||||||||
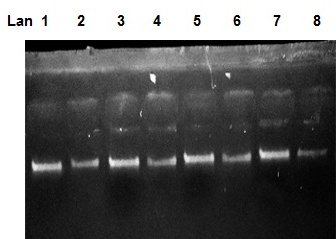 | Figure 1. Chromosomal DNA bands on 1% agarose gel at 100 volt for 20min. DNA sample were extracted from some PCOS women |
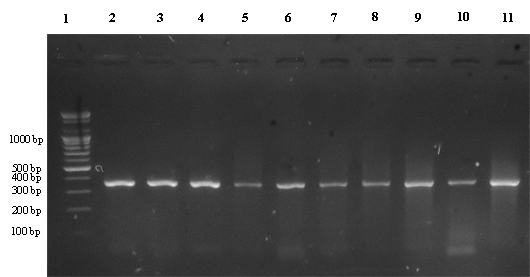 | Figure 2. PCR products of (INS-promoter) region on 2% agarose gel at 100 voltages for (35-50) min |
|
4. Conclusions
- 1- Polycystic ovaries syndrome can be considered as a complex metabolic syndrome triggered by the interact effect of genetic and environmental factors.2- There was an association between Polycystic ovaries syndrome (PCOS) and insulin resistance because the insulin plays an important role (direct and indirect) in reproductive function.3- By carrying out the DNA sequencing, many mutations (substitution, deletion and insertion) have been reported in insulin gene (INS-promoter) which play an essential role in insulin expression.
References
| [1] | Fauser, B.; Diedrich, K.; Bouchard, P.; Dominguez, F.; Matzuk, M. and Franks, S. (2011). "Contemporary genetic technologies and female reproduction" in. Human Reproduction Update, 17(6). Pp:829–847. |
| [2] | Aittomaki, K.; Lucena, J.; Pakarinen, P.; Sistonen, P.; Tapanainen, J. and Gromoll, J. (1995). Mutation in the follicle stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell; 82. Pp: 959–968. |
| [3] | Azziz, R.; Woods, K.; Reyna, R.; Key, T.; Knochenhauer, E. and Yildiz, B.O. (2004). The Prevalence and Features of the Polycystic Ovary Syndrome in an Unselected Population. J Clin Endocrinol Metab, 89. Pp:2745‐2749. |
| [4] | Baillargeon, J. and Nestler. J. (2006). Polycystic Ovary Syndrome: A Syndrome of Ovarian Hypersensitivity to Insulin? J Clin Endocrinol Metab. 91:Pp:22‐24. |
| [5] | Poretsky, L.; Clemons, J. and Bogovich, K. (1992). Hyperinsulinemia and human chorionic gonadotropin synergistically promote the growth of ovarian follicular cysts in rats. Metab.41,Pp :903‐910. |
| [6] | Desai, M.; Zeggini, E.; Horton, V.; Owen, K.; Hattersley, A.; Levy, J. (2006). The variable number of tandem repeats upstream of the insulin gene is a susceptibility locus for latent autoimmune diabetes in adults. Diabetes.55(6), Pp:1890-1894. |
| [7] | Sambrook, J. (1989). Molecular Cloning: A Laboratory manual. Second Edition. (Plainview, New York: Cold Spring Harbor Laboratory Press). |
| [8] | Pugliese, A. and Miceli, D. (2002). The insulin gene in diabetes. Diabetes MetabRes Rev, 18.Pp:13-25. |
| [9] | Brian, H. (2004). The Burden of Diabetes in Indiana, Ind. State Dept. of Health Diabetes Prevention and Control Prog. |
| [10] | Knochenhauer, E.; Key, T.; Kahsar-Miller, M.; Waggoner, W.; Boots, .L. and Azziz, R. (1998). Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 83, Pp:3078–3082. |
| [11] | Yuping, X.; Zhaolian, W.; Zhiguo, Z.; Qiong, N.; Pin, H. and Xiaohui, Z. (2009). No association of the insulin gene VNTR polymorphism with polycystic ovary syndrome in a Han Chinese population. J. Reprod Bio & Endocrinol. 7:141. |
| [12] | Mahdi, S. (2012). Hormonal-Genetical Study on Women with Polycystic Ovary Syndrome and Thyroid Disorders. A thesis Submitted to the Counsel of Genetic Engineering and Biotechnology Institute for Postgraduate Studies University of Baghdad. |
| [13] | Hamilton-Shield, J. (2007). Overview of neonatal diabetes. Endocr Dev.12, Pp: 12 –23. |
| [14] | Flanagan, S.; Patch, A.; Mackay, D.; Edghill, E.L.; Gloyn, A.; Robinson, D. (2007). Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 56, Pp:1930 –1937. |
| [15] | Bennett, S.; Lucassen, A. and Gough, S. (1995). Susceptibility to human type 1 diabetes at IDDM2 is determined by tandem repeat variation at the insulin gene minisatellite locus. Nuffield Department of Surgery, The Wellcome Trust Centre for Human Genetics, University of Oxford, Windmill Road, Oxford .UK. |
| [16] | Hastings, P.; Lupski, J.; Rosenberg, S. and Ira, G. (2009). Mechanisms of change in gene copy number. Nat Rev. Genet. 10 (8), Pp: 551 564. |
| [17] | Carroll, S.; Grenier, J. and Weatherbee, S. (2005). From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. Second Edition. Oxford: Blackwell Publishing. |
| [18] | Malecki, M. (2005). Genetics of type 2 diabetes mellitus. Diabetes Res Clin Pract. 68, Pp:10–21. |
| [19] | Kennedy, G. German, M. and Rutter, W. (1995). The minisatellite in the diabetes susceptibility locus IDDM2 regulates insulin transcription. Nat Genet 9:293 – 298. |
| [20] | Sabater, L.; Ferrer-Francesch, X.; Sospedra, M.; Caro, P.; Juan, M. and Pujol-Borrell, R. (2005). Insulin alleles and autoimmune regulator (AIRE) gene expression both influence insulin expression in the thymus. J Autoimmun. 25, Pp:312–318. |
| [21] | Vafiadis, P.; Bennett, S.; Todd, J.; Nadeau, J.; Grabs, R. and Goodyer, C. (1997). Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet . 15,Pp:289-292. |
| [22] | Day. I.; Rodriguez. S.; Kralovicova. J.; Wood. P.; Vorechovsky. I.; and Gaunt. T.R. (2006). Questioning INS VNTR role in obesity and diabetes: subclasses tag IGF-INS-TH haplotypes; and −23 HphI as a STEP (splicing and translational efficiency polymorphism). Physiol Genomics .28 (11). |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML