-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(2): 99-104
doi:10.5923/j.ajmms.20150502.07
A Review on Lozenges
Stephen O. Majekodunmi
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Uyo, Uyo, Nigeria
Correspondence to: Stephen O. Majekodunmi, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Uyo, Uyo, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Lozenges are one of the widely used solid dosage forms. They contain medicament and are meant to be in mouth or pharynx. Lozenges have been in use since 20th century and are still in commercial production. Lozenges provide a palatable means of dosage form administration and possess excellent advantages; though they suffer certain disadvantages too. Lozenges are adopted for both local and systemic administrations and a wide range of active ingredients can be incorporated in them. Lozenges currently available in market are: Caramel based soft lozenges, hard candy lozenges and compressed tablet lozenges. This present review covers researches performed till date, formulation and evaluation parameters, packaging and applications of lozenges.
Keywords: Lozenge, Medicament, Molding, Troches, Pastilles
Cite this paper: Stephen O. Majekodunmi, A Review on Lozenges, American Journal of Medicine and Medical Sciences, Vol. 5 No. 2, 2015, pp. 99-104. doi: 10.5923/j.ajmms.20150502.07.
Article Outline
1. Introduction
- Oral dosage forms vary and have advantages over other dosage forms. They are economical and safe to the patient. They are the most natural and easiest route of drug administration. No nursing is required, which means the patient can take it with no help. Their toxicity is delayed due to the onset of action which permits easier recovery than in case of other dosage forms. They are appropriate for any patient, whatever the age is. Oral dosage forms have disadvantages as well. They are not the first choice of drugs if the patient suffers chronic vomiting. They are not good choice in case of uncooperative patients as children and infants. They are not suitable in emergency and for unconscious patients. They are not convenient for a patient with a gastrointestinal disorder such as diarrhea, constipation, ulceration, hyperacidity in stomach. They are not convenient if a patient suffers malabsorption syndrome in which absorption through small intestine is not ensured. Oral dosage forms are not adequate for medications liable to inactivation or destruction in the GIT. e.g. insulin is a protein, if taken orally, it is digested in the stomach like the protein present in food such as meat and fish. Sometimes, the medication itself is the cause of such problems in the GIT. like aspirin and many NSAIDs which may lead to ulcers in stomach on recurrent usage in the long run. Lastly, there is delayed onset of action because absorption takes time. Lozenges are oral solid preparations that are intended to be dissolved inside the mouth or pharynx. They may contain one or more medicaments in a flavoured and sweetened base and are intended to treat local irritation or infection of mouth or pharynx and may also be used for systemic drug absorption [1] [2]. Lozenges are intended to achieve local effect as soothing and purging the throat. Sometimes they are used to relieve cough. Lozenges are also for systemic effect provided the drug is well absorbed through the buccal linings or when it is swallowed [3]. Lozenges are placed in oral cavity. Since the sublingual lozenges may be impractical due to their size, buccal lozenges are formulated and have been extensively used and are intended to be placed between the cheek and the gums. Though the lozenge dissolution time is about 30 minutes, this depends on the patient; as the patient controls the rate of dissolution and absorption by sucking on lozenge until dissolves. The consequence of this can be high variabilities in amounts of drug delivered each time the lozenge is administered. Sucking and the subsequent production of saliva may also lead to increased dilution of the drug and accidental swallowing [4].There are various types of lozenges, namely: (A) Nicotine lozenges are used for helping smokers to quit smoking. It is a smoking deterrent and works by providing low levels of nicotine, which may help one to quit smoking by lessening the physical symptoms of withdrawal. Nicotine should not be used if: 1) if one is allergic to any ingredient in nicotine lozenges. 2) One who has had a recent heart attack. 3) One who has severe or worsening chest pain or a severe irregular heartbeat. Some medical conditions may interact with nicotine lozenges, e.g. Acetaminophen, adrenergic antagonists (e.g. prazosin), beta-blocker (e.g labetalol, propranolol), caffeine, insulin, oxazepam, pentazocine, theophylline, or tricyclic antidepressants (e.g. imipramine) because the risk of their side effects may be increased when one stops smoking.(B) Linctagon lozenges contain the active ingredient Pelargonium sidoides, and Eucalyptus oil. It provides soothing support for coughing and a sore and irritated throat. It is suitable for diabetes.(C) Fungilin lozenges are used to treat infections in or around the mouth, throat or tongue caused by yeast like fungi called Candida. This condition is known as candidiasis or thrush. Candida albicans is the most common cause of thrush.(D) Flurbiprofen lozenges: Flurbiprofen belongs to the group of medicines known as NSAIDs. These medicines are used to relieve pain and inflammation. The lozenges are used to ease the symptoms of a sore throat.(E) Low-dose natural human interferon-alpha lozenges are used in the treatment of Behcet’s syndrome. There had been evidence that low-dose local IFN could be beneficial in the management of recurrent oral ulcers. (F) Actiq lozenges: These lozenges contain the active ingredient fentanyl, which is a type of medicine called an opioid analgesic (painkiller). The opioids are a group of very strong painkillers that are related to morphine. People with long-term, ongoing, severe pain, such as the pain caused by cancer, are given opioid medicines as painkillers. However, occasionally the pain can become worse despite taking these strong painkillers. This is known as “breakthrough” pain. Actiq lozenges are used to relieve this “breakthrough” pain in people already receiving opioid painkillers. The lozenges consist of a lozenges attached with edible glue to handle. The lozenge is placed in the mouth next to the cheek and moved around the mouth using the handle. The medicine is rapidly absorbed into the blood through the mucosal skin on the inside of the mouth to provide rapid relief from pain. It is used for relieving breakthrough pain in people taking regular opioid painkillers for long-term, ongoing, severe pain due to cancer. (G) Zinc gluconate lozenges and zinc acetate lozenges: As it is known, zinc is an essential mineral and antioxidant found in every body cell. It is essential for a wider range of physiological functions and is an important cofactor for more than 200 enzymes, more than any other mineral. Researchers have studied the use of zinc as a way to treat or reduce symptoms of the cold virus. The research found that taking zinc, either as syrup or lozenges, through the first few days of a cold may shorten the length of the illness. It also appeared to prevent colds in people who used it over the course of about five months. So, for now the study results on using zinc as a cold remedy are inconclusive. For every study showing a positive benefit with zinc, there’s another study showing no benefit at all. Many experts say that if there is any benefit in taking zinc or zinc lozenges, it is very minor. There are a few important factors about the use of zinc for the common cold. One is that the formulation (gluconate, sulfate, acetate) and some flavoring additives may make difference; this detail is still being worked out by our researchers. A study says that zinc may be effective at reducing the duration of symptoms of the common cold when taken at early onset and at a dose greater than 75mg per day. Evidence suggests that several possible mechanisms may make zinc an effective treatment. But the mechanisms of action of this substance in treating the common cold remain unknown. Zinc ions are known to inhibit replication of common cold viruses. Zinc is also reported to control recurrent herpes simplex virus skin infections. Evidence exists that clearly shows zinc gluconate lozenges to be effective in reducing duration of common colds.So now the only question is by how much, which depends on ZIA values. Zinc acetate is extremely soluble and is 3.33 times as ionizable as zinc gluconate. Equivalent amounts of zinc from zinc acetate produce higher ZIA values and better results against common colds than zinc gluconate. So, because of superior properties, zinc acetate is the successor to zinc gluconate in lozenges to be used for treating or curing the common cold. Nowadays, two basic zinc acetate lozenges formulations are recommended for use in treating or curing commons, namely the standard design and the advanced design. The standard design relies on Saccharin to provide added sweetness to dextrose tablet base. The advanced design relies on fructose to provide the necessary added sweetness.Depending on the type of lozenges, they may be prepared by molding or by compression. Molded lozenges are called pastilles while compressed lozenges are called troches [2].Lozenges should dissolve slowly in mouth and possess some degree of smoothness; with their shape being without corners. Lozenges may be formulated with various shapes, like flat, circular, octagonal, biconvex or bacilli, meaning short rods or cylinders [2].
2. Advantages and Disadvantages of Lozenges
- Lozenges offer many advantages to formulation pharmacists. They can be given to those patients who have difficulty in swallowing [4]; easy to administer to geriatric and pediatric population; pleasant taste; It extends the time of drug in the oral cavity to elicit a specific effect; easy to prepare, with minimum amount of equipment and time; do not require water intake for administration; technique is non invasive, as is the case with parenterals. Lozenges also offer certain disadvantages. Some of which are: It could be mistaken as candy by children, hence should be kept out of the reach of children because of the non ubiquitous distribution of drug within sativa for local therapy.
3. Medicament Incorporated into Lozenges
- Drug candidates which can be incorporated in lozenges include antiseptics, local anesthetics, antibiotics, antihistamines, antitussives, analgesics, decongestants and demulcents.
4. Classification of Lozenges
4.1. According to Site of Action
- Lozenges can be classified into various classes based on various methods such as according to the site of action which can either be local and systemic effects. Examples of local effects are antiseptics, decongestants, while vitamins and nicotine are examples of systemic effect.
4.2. According to Texture and Composition
4.2.1. Chewy or Caramel Based Medicated Lozenges
- Chewy or caramel based medicated lozenges are the dosage form in which medicament is incorporated into a caramel base which is chewed instead of being dissolved in mouth. Most formulations are based on the glycerinated gelatin suppository formula which consists of glycerin, gelatin, and water. These lozenges are often highly fruit flavored and may have a slightly acidic taste to cover the acrid taste of the glycerin Its constituent ingredients are the candy base, whipping agent, humectants, lubricants, flavor and of course medicaments incorporated into the lozenges. The candy base consists of a mixture of sugar and corn syrup in a ratio of 50:50 to 75:25 sugar to corn syrup. The whipping agents are used to incorporate air in toffee-based confections to obtain the desired degree of soft chew. These are exemplified by milk protein, egg albumin, gelatin, xanthan gum, starch, pectin, algin and carageenen. The humectants improve chew and mouth feel properties and include glycerin, propylene glycol and sorbitol. Lubricants are added to avoid sticking of candy to the teeth while chewing. It includes vegetable oils and fats. Medicaments up to 35- 40% can be incorporated. Seeding crystal involves addition of fine powdered sugar at 3-10% to warm candy mass to speed up the crystallization and allow the base to be formed into tablets in a much shorter time. Seeding crystals involves addition of fine powdered sugar at 3-10% to warm candy mass to speed up the crystallization and allow the base to be formed into tablets in a much shorter time [5].
4.2.1.1. Manufacturing of Chewy or Caramel Based Medicated Lozenges
- The candy base is cooked at 95-125℃ and transferred to planetary or sigma blade mixer. Mass is allowed to cool to 120℃. This is followed by the addition of whipping agent below 105℃. The medicaments are then added between 95-105℃. Color is dispersed in humectant and added to the above mass at a temperature above 90℃. Seeding crystals and flavour are then added below 85℃ followed by lubricant addition above 80℃. Candies are then formed by rope forming.
4.2.2. Compressed Tablet Lozenges
- If the active ingredient is heat labile, it may be made into lozenge by compression. The granulation is prepared in a manner similar to that used for any compressed tablet. The lozenge tablets differ from conventional tablets in terms of organolepticity, non-disintegrating characteristics and slower dissolution profiles [2] [6].The lozenge is made using heavy compression equipment to give a tablet that is harder than usual, as it is desirable for the troche to dissolve slowly in mouth. They are usually flat faced with sizes, weight, hardness, and erosion time ranging between, 5/8-3/4 inch, 1.5-4 g, 30-50 kg inch2 and 5-10 min, respectively. The ingredients for compressed tablet lozenges are tablet based or vehicles which are sugar such as dextrose, sucrose. Other vehicles are sugar free vehicles such as mannitol, sorbitol, polyethylene glycol (PEG) 6000 and 8000. Some commercially available sugar based vehicles include- Emdex, Nu-tab, Sweetrex, Mola-tab, Hony-tab, Sugartab. Other fillers include dicalcium phosphate, calcium sulphate, calcium carbonate, lactose and microcrystalline cellulose. Binders are also included to hold the particles of mass as discrete granules and include acacia, corn syrup, sugar syrup, gelatin, polyvinyl-pyrrolidone, tragacanth and methylcellulose. Lubricants are used to improve flow of final troche mixture and include magnesium stearate, calcium stearate, stearic acid and PEG. The colours used include water soluble and lakolene dyes.
4.2.2.1. Manufacturing of Compressed Tablet Lozenges
- Manufacturing of compressed tablet lozenges can either be direct compression and wet granulation. In direct compression, ingredients are thoroughly mixed and then compressed. In wet granulation, sugar content is pulverized by mechanical comminution to a fine powder (40-80 mesh size). Medicament is added and thoroughly blended. The blended mass is subjected to granulation with sugar or corn syrup and screened through 2-8 mesh screen. This is followed by drying and milling to 10-30 mesh size. Flavour and lubricant are then added prior to compression.
4.2.3. Soft Lozenges
- They are either meant for chewing or for slow drug release in mouth. They can be made from PEG 1000 or 1450, chocolate or sugar-acacia base while some soft candy formulations can also contain acacia and silica gel. Acacia is used to provide texture and smoothness and silica gel is used as a suspending agent to avoid settling of materials to the bottom of the mold cavity during the cooling. The formulation requires heating process at about 50℃, hence is only suitable to heat resistant ingredients. These are mixtures of sugar and other carbohydrates in an amorphous (non crystalline) or glassy state. They can also be regarded as solid syrups of sugars.The moisture content and weight of hard candy lozenge should be in between, 0.5 - 1.5% and 1.5-4.5 g respectively. These should undergo a slow and uniform dissolution or erosion over 5- 10 min., and they should not disintegrate. The disadvantage of this method is that the temperature required for their preparation is high hence heat labile materials cannot be prepared [2] [4].
4.2.3.1. Manufacturing of Soft Lozenges
- On the account of the soft texture of these lozenges, they can be hand rolled and then cut into pieces or the warm mass can be poured into a plastic mold. Mold cavity should be overfilled if PEG is used, as PEG's contract as they cool. This is not required in case of chocolate as it does not shrink [4].Phaechamud and Tuntarawongsa [7] fabricated clotrimazole soft lozenges by molding method and evaluated the factors that affect the physical properties of lozenge. They found that increasing amounts of PEG 1500, xanthan gum or xylitol increased the hardness of the lozenge. And also disintegration time was found to be increased on increasing amount of actives and hardness [6].
4.2.4. Hard Candy Lozenges
- Hard candy lozenges are mixtures of sugar and other carbohydrates in an amorphous (noncrystalline) or glassy state. They can also be regarded as solid syrups of sugars. The moisture content and weight of hard candy lozenge should be between, 0.5 to 1.5% and 1.5-4.5g respectively. These should undergo a slow and uniform dissolution or erosion over 5-10min., and should not disintegrate. The temperature requirements for their preparation is usually high hence heat labile materials cannot be incorporated in them [1] [6]. The ingredients for hard candy lozenges include body agent or base which is corn syrup that is available on Baume basis. A 43° Baume corn syrup is preferred in hard candy lozenges. Sweetening agents such as sucrose, dextrose, maltose and lactose are added. Acidulents are added to candy base to strengthening the flavor characteristics of the finished product. Commonly used acids are citric, tartaric, fumaric and malic acid. Colours include FD & C colours, orange colour paste, red colour cubes etc while flavours used include menthol, eucalyptus oil, spearmint, cherry flavor etc. Medicaments up to 2-4% can be incorporated in the hard candy lozenges. Salvage solution can be liquid or solid [1].
4.2.4.1. Manufacturing of Hard Candy Lozenges
- The candy base is cooked by dissolving desired quantity of sugar in one third amount of water in a candy base cooker. This is continued till the temperature rises to 110℃. Corn syrup is added and cooked till the temperature reaches 145-156℃. The candy mass is removed from the cooker and transferred to a lubricated transfer container mounted onto a weight check scale where the weight of the mass is checked. This is followed by color addition in form of solutions, pastes or color cubes. The mass is then transferred to a water-jacketed stainless steel cooling table for mixing and the flavor, drug and ground salvage is added. The mass is either poured in mold or pulled into a ribbon while cooling and then cut to desired length. The obtained lozenges are packaged [2] [6]. Cocaine voice tablet lozenges and pastilles were developed in late 1800's and were indicated in Extra Pharmacopoeia, 1888. They were used by singers and public speakers for the remedy of vocal huskiness and hoarseness [1].Esimone et al., [8] formulated and evaluated antimicrobial activity of herbal lozenge of garlic and ginger extract by molding method. The antimicrobial activity was evaluated against Candida albicans, E.coli and Staphylococcus aureus using Nystatin as standard. The formulation inhibited growth of laboratory strains of C.albicans but not S.aureus and E.coli and hence concluded that lozenges can be used in non-resistant oral thrush.Greey et al., [9] prepared penicillin agar pastilles. In order to prolong the retention time they tried gelatin, gelatin+agar and agar combinations with penicillin whose retention times were found to be 1hr, less than 1hr and 4-5hrs, respectively. This formulation has already been used in fuso spirochaetal infection treatment and is being studied for hemolytic streptococcal infection treatment of throat.A multicenter, randomized, double blind, single dose study was carried out by Wade et al., [10] for assessing the efficacy of amylmetacresol and 2,4 – dichlorobenzyl alcohol (AMC/DCBA) warm and cool lozenge for the relief of acute sore throat. Analgesic and sensorial benefits of AMC/DCBA warm and cool lozenge was compared against unflavored non-medicated placebo lozenge and significant analgesic, functional sensorial and emotional effects against the placebo were observed. Sore throat relief, difficulty in swallowing and throat numbness were observed, also emotional benefits included happiness, better feel and less frustration [10].Shukla et al. [11] evaluated in vivo behaviour of controlled and pulsatile release pastilles for the treatment of asthma, chronic obstructive pulmonary disease, (COPD) and for chrono-therapeutic management of nocturnal asthma. They found that, enteric coating of pastilles delayed the in vivo drug release and can be used in nocturnal asthma.
5. Quality Control of Lozenges
5.1. In Process Quality Control of Candy Base Manufacturing
- As the candy base manufacture is commenced, a check on following parameters is performed: corn syrup and sugar delivery gears; temperature, steam pressure and cooking speed of precookers and temperature, steam pressure, cooking speed and vaccum of candy base cookers [1].
5.2. Moisture Analysis
- Gravimetric, Karl Fisher titration and Azeotropic distillation methods are used to determine the moisture content of lozenges. In gravimetric method, sample (1g) is weighed and placed in vaccum oven at 60-70℃ for 12-16hrs. Final weight is subtracted from initial and the difference in moisture content is calculated. Karl Fischer titration involves calculating a sample to contain 10-250mg water in titration flask and titrated with Karl Fischer reagent. In azeotropic distillation method, 10- 12g candy is pulverized and placed in 500ml flask to which 150-200ml toluene is added. Flask is connected to a reflux condenser and is refluxed for 1-2hrs. Water collected gives the amount of water present in the sample [1].
5.3. Determination of Sugar and Corn Syrup Ratios
- This is performed by "Dextrose equivalent method and Lane Eynon Titration method".2
5.4. Determination of Percentage of Reducing Sugars
- Standard anhydrous dextrose (3g) is dissolved in 500ml water. The solution is boiled for 2 min and 2 drops of methylene blue is added and titrated against 25 mL of alkaline cupric tartrate solution (Fehling's solution) to a yellowish red end point [1].
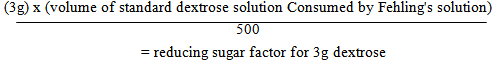 | (1) |
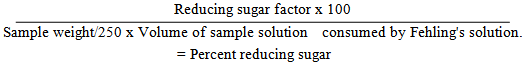 | (2) |
5.5. Physical and Chemical Testing for Lozenges
- Hardness of lozenges is determined by Pfizer or Monsanto hardness tester [12] [13] while diameter and thickness are determined by vernier caliper. The Fourier transform Infrared Spectroscopy (FTIR) determines the drug excipient interaction studies while friability is determined by Roche Friabilator operated at 25rpm for 4min [12]. In weight variation determination, 20 lozenges are weighed and average weight determined; individual weight is compared to the average weight. In-vitro drug release is carried out in USP II paddle type dissolution apparatus [13]. In drug content determination, appropriate number of lozenges are crushed and dissolved in an appropriate solvent and the absorbance of the solution is measured spectrophotometrically.
5.6. Microbial Check on Lozenges
- In this microbial check, the presence of any bacterial, mold or spore contamination is checked in raw materials, finished products, machinery, cooling tunnels, environmental conditions and storage drums. Laboratory microbial testing should include the following counts: total plate, total coliform, yeast and mold, E.coli, Staphyllococcus, Salmonella [1].
5.7. Stability Testing for Lozenges
- Lozenges are subjected to stability testing under following conditions: 2 months at 60℃, 3-6months at 45℃, 9-12 months at 37℃, 36-60 months at 25℃ [1]. Lozenges in their final packs are subjected to the following conditions for stability testing: 25℃ at 80% relative humidity (RH) for 6-12 months, 37℃ at 80% RH for 3 months, 25℃ at 70% RH for 6-12 months [14].
5.8. Packaging of Lozenges
- Since the lozenges are hygroscopic in nature, a complex and multiple packaging is adopted. The individual unit is wrapped in polymeric moisture barrier material which are then placed in tight or moisture resistant glass, polyvinyl chloride or metal container that is over wrapped by aluminum foil or cellophane membrane [14].
5.9. Storage of Lozenges
- Lozenges should be stored away from heat and out of reach of children. They should be protected from extremes of humidity. Depending upon the storage requirements of both, the drug and the base, either room temperature or refrigerator temperature is usually indicated [14].
5.10. Applications of Lozenges
- Lozenges are employed for the treatment of local as well as systemic disorders. A variety of drug candidates can be incorporated in them for the treatment of and relief from conditions of oral as well as throat infections such as oral thrush, sore throat, cough, gingivitis, pharyngitis, decongestant, etc. Moreover these also have been used to deliver the drug systemically for smoking cessation and pain relief.
6. Conclusions
- Lozenges are medicated confections that have been developed about 20th century ago and are still under commercial production. Most of the preparations are available over the canter products and are very economic dosage forms. They are designed for local as well as systemic therapy. A wide range of actives can be incorporated within their structure. Lozenges enjoy an important position in pharmacy and will continue to remain so in future.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML