-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(2): 59-62
doi:10.5923/j.ajmms.20150502.01
The Impacts of in Vitro Improving of Seminal Plasma Quality on Sperms Motility of Asthenospermic Infertile Samples
Ghassan Th. Saeed1, Nawal Khairy Hussain AL-Ani2, Ban Thabit Saeed2
1College of Medicine, University of Baghdad, Iraq
2High Inst. of Infert. Diag. & Art. Al-Nahrain University, Baghdad, Iraq
Correspondence to: Nawal Khairy Hussain AL-Ani, High Inst. of Infert. Diag. & Art. Al-Nahrain University, Baghdad, Iraq.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Motility of the sperm is the most important independent function of the sperm to reach the oocyte and complete fertilization. The seminal plasma is the media for sperm that contain the nutrient and some activation and inhibition factors. Many studies tried to explain asthenospermia (defective sperm motility) according to findings in the seminal plasma. The aim of this study is to evaluate the effects of improving of seminal plasma quality on sperms motility of asthenozoospermic semen samples.Sperms of 100 seminal samples of infertile patients with asthenospermia with the seminal plasma of 100 healthy normal samples were incubated for 60 minute at 37C° and studied again. This study showed that incubation of sperms with poor motility in a media made from seminal plasma of healthy samples significantly improve the motility and decrease the percentage of immotile sperms in the diseased samples.
Keywords: Asthenospermia, Seminal plasma, Sperm activation
Cite this paper: Ghassan Th. Saeed, Nawal Khairy Hussain AL-Ani, Ban Thabit Saeed, The Impacts of in Vitro Improving of Seminal Plasma Quality on Sperms Motility of Asthenospermic Infertile Samples, American Journal of Medicine and Medical Sciences, Vol. 5 No. 2, 2015, pp. 59-62. doi: 10.5923/j.ajmms.20150502.01.
1. Introduction
- The spermatozoa are smallest cells in the body that have to fulfil their functional extracorporeal. To perform this, it has a highly efficient mechanism to generate motility and hence, sperm motility is the only natural way through which sperm meet the ova during fertilization [1]. Mammalian spermatozoa are produced in testes and then transferred to epididymides for maturation and storage. It has been considered that most mammalian spermatozoa in the epididymal fluids are mature but nonactivated [2]. The activation of motility of epididymal spermatozoa would not take place unless the spermatozoa were mixed with male accessory gland secretions at the time of ejaculation, or diluted into a buffer solution containing activating factor, depending on type species [3]. Asthenospermia means reduce sperm motility or progressive motility or both [4], and considered one of the most important factors related to male infertility [5]. The seminal fluid is a complex medium containing a great variety of molecules, mainly produced by sex accessory glands, and also cells other than spermatozoa (e.g. leucocytes). This fluid may contain factors that modified the state of sperms motility like antisperm antibodies or infection [6]. Asthenospermia may also be resulted from a defect in the sperm structure and this is called primary asthenospermia and usually involved high percentage of the ejaculated sperms and the methods of assisted reproductive techniques is the only treatment. While the secondary causes could be of idiopathic causes, prolonged abstinence period, or partial ductal obstruction. Sometimes, drug therapy may result in decreased motility and this can be improved by the removal of the drug, or introduction of specific culture media to the sperm washing procedure [7]. Asthenozoospermia may be managed by in vitro sperm activation using different types of stimulators [8], and then in intrauterineinsemination or in vitro fertilization [9].In this study, we tried to prove that the healthy seminal plasma had properties and dissolved factors that improve sperm motility.
2. Samples and Methods
- This study was done in the seminal fluid analysis lab in the High institute of infertility diagnosis and assisted reproductive techniques AL-Nahrain University \ Baghdad \ Iraq in the period from September 2014 to February 2015.Patient included in this study were with history of primary infertility and with regular unprotected sexual contacts and no abnormality in physical and hormonal examination.The samples were collected in a next private room by masturbation after at least 72 hours of sexual abstinence. Ejaculates were allowed to liquefy at 37℃ for 25 minutes up to 40 minutes before analysis. Manual semen analysis was performed according to World Health Organization (WHO) guidelines to determine sperm concentration and motility. Then samples with asthenospermia (poor sperm motility) were selected with accepted count and structural character to be used in this study.Then the samples were re-examined using Computer assisted sperm analysis (CASA) which a digital system designed for automatic analysis of the sperm concentration, motility, and morphology.Sperms of selected 100 seminal samples with asthenospermia with the seminal plasma of 100 healthy normal samples were used in this study. The selected seminal sample was centrifuged for 10 min at 3000 revolution per minute(rpm) then the plasma was removed. At the same time a healthy sample with a good count and motility was also centrifuged for 10 min at 3000 rpm in the same way but the plasma will be taken to be centrifuged again for another 10 minutes then the plasma will mixed with the sperms of the asthenospermic sample and incubated for 60 minute at 37℃ to be studied again by the same examiner and the same instruments.Patient’s agreement was taken and all the samples were discarded after finishing the study and the patients were informed about the results of studying their samples.
3. Statistical Analysis
- Statistical analysis was performed by the SPSS 20 statistical software program. Univariate data were summarized using standard descriptive statistics, tabulation of categorical variables and numerical variables. An association between categorical variables was assessed via paired T-test. In all statistical analyses, a p value < 0.05 was considered as significant.
4. Results
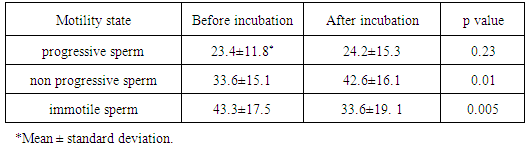
- There was non-significant increase in the percentage of sperms with active progressive motility while there was a significant increase in the percentage of sperms with sluggish non-progressive sperms. Also there was a significant decrease in the percentage of immotile sperms after treatment.
|
5. Discussion
- This study showed that incubation of sperms with poor motility in a media made from seminal plasma of healthy samples (natural media) significantly improve the motility and decrease the percentage of immotile sperms in the diseased samples and that means that there was a significant number of sperms that changed their character of motility from "immotile" to motile (but sluggish) sperms. Also there was an increase in the percentage of progressive motile sperms in the treated sample but not significant. The motility could be structural dysfunction if the sperm was of abnormal shape or tail but in this study we tried to select samples with accepted normal character percentages and the abnormal motility are of unknown cause. Many studies showed the effects of adding certain drugs or electrolytes as chemical activation [10] or heat [11], vibration [12], and laser [13] as physical activation factors and many media nowadays are available for treating defective samples before performing assisted reproductive techniques especially before intrauterine insemination or in vitro fertilization. Despite the development of science and increasingly sophisticated diagnostic methods, in some cases, the etiology and pathogenesis of male infertility are still unknown and constitute the group of idiopathic infertility. Male fertility disorder is attributed to environmental factors such as exposure to certain chemicals, heavy metals, pesticides, and heat, or electromagnetic radiation [14]. Smoking, alcohol abuse, chronic stress, obesity, urogenital trauma, and inflammation in the male reproductive system are also associated with decreased male fertility [15]. The consequence of most of these factors is oxidative stress. Oxidative stress results from the imbalance between production of the reactive oxygen species (ROS) and the protective effect of the antioxidant system responsible for their neutralization and removal. An excess of ROS causes a pathological reaction resulting in damage to cells and tissues. Spermatozoa are particularly vulnerable to the harmful effects of ROS. Oxidative stress affects their activity, damages DNA structure, and accelerates apoptosis, all of which consequently decrease their numbers, hinders motility and development of normal morphology, and impairs function [16]. This might leads to disturbances in fertility or embryo development disorder. The main cellular sources of ROS in the semen are immature sperm cells and white blood cells. The increase in the number of leukocytes may be due to infection and inflammation, but can also be secondary to harmful environmental factors, long sexual abstinence, or varicocele [17]. The protective antioxidant system in the healthy semen is composed of enzymes, as well as non-enzymatic substances, which closely interact with each other to ensure optimal protection against ROS. Non–enzymatic antioxidants include vitamins A, E, C, and B complex, glutathione, pantothenic acid, coenzyme Q10 and carnitine, and micronutrients such as zinc, selenium, and copper. It seems that a deficiency of any of them can cause a decrease in total antioxidant status and many studies shows that these factors are of abnormal levels in abnormal samples [18] but still need further understanding. This result of this study disagreed with the hypothesis that state that idiopathic asthenospermia is a structural abnormality rather than a functional defect even in normal looking sperms [19].It is well known that the mitochondria in the sperm midpiece are the energy generator for mammalian sperm and sperm require a sufficient supply of adenosine triphosphate from mitochondrial oxidative phosphorylation for normal function [20] and each mature mammalian sperm contains approximately 50–75 mitochondria and one copy of mtDNA in each mitochondrion in midpiece [21] and this number of copy was found to be different in further studies [22, 23]. Kao and his team have demonstrated multiple deletions of mtDNA in human sperm with low motility scores [24, 25]. Sun and his co-workers in 2007 studied the ultrastructure of 151 samples with idiopathic asthenospermia and found that there were various mitochondrial pathological changes that exist in idiopathic asthenospermia and there is a close association between the sperm viability and mitochondrial ultrastructure and mitochondrial function [26]. The present study concluded that the healthy seminal plasma contain what we can called "curing factors" that improve the motility the sperms that could be functional problem rather than structural abnormality. This study suggested further studies on each seminal plasma elements to find the optimal media that resample the natural seminal plasma because the usage of any seminal part in treating another diseased seminal fluid and using it in assisted reproductive techniques might be not accepted socially and religiously.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML