-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(1): 42-47
doi:10.5923/j.ajmms.20150501.08
Effects of GM1 Ganglioside on the Recovery of Spatial Learning after a Lesion of the Nucleus Basalis Magnocellularis in an Experimental Model of Alzheimer’s Disease in Adult Male Rats
Sedigheh Ashkavandi 1, Ahmad Ali Moazedi 1, Saeed Semnanian 2, Hooman Eshagh Harooni 1, Mahdi Pourmehdi 3
1Dept. of Biology, Faculty of Science, Shahid Chamran University, Ahvaz, Iran
2Dept. of Physiology, Tarbiat Modarres University, Tehran, Iran
3Dept. of- Food hygiene, Faculty of Veterinary medicine, Shahid Chamran University, Ahvaz, Iran
Correspondence to: Sedigheh Ashkavandi , Dept. of Biology, Faculty of Science, Shahid Chamran University, Ahvaz, Iran.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Monosialoganglioside GM1 is a sialic acid containing the glycosphingolipid found in the plasma membrane of most vertebrate cells, particularly in the nervous system. In the present study, the effects of GM1 on the spatial learning and memory of Alzheimer’s rats were investigated. Thirty-five male rats were randomly allocated into five groups: Control group, Lesion group were received lesion with electrically method: 0.5mA, 3s (bilaterally lesion of Nucleus Basalis Magnocellularis (NBM) to create Alzheimer’s model), Sham group (entering the electrode in to the NBM without lesion), GM1 group (lesion+ 2 mg/kg GM1) and saline group (lesion NBM + 0.2 ml saline). One week after, the rats were training with Y-maze task within five days. The animals were tested for memory one month later. The data were recorded to comparing between five groups. One way ANOVA test showed that time, group and the mutual effect of time and group had a significant effect on learning (P<0.001). So that the LSD test showed there was a significant difference between the Control and Lesion groups (P<0.001), it means that the lesion of NBM comparing the control group, had reduced the spatial learning of animals, Lesion and Sham (P<0.05), means that entering electrode without any electrical induction had no effect on the rat’s spatial learning, Saline and GM1 (P<0.001), suggesting that the injection of GM1(2mg/kg) increased the spatial learning while saline had no effect. There were no significant difference between the results in the memory test and fifth day (P>0.05), it means that nor GM1, saline and the lesion of NBM had no effect on the spatial memory. The present finding showed that the mutual lesion of NBM has reduced the spatial learning and the GM1 has a positive effect on spatial learning in Alzheimer’s rats and enhanced the learning in these animals.
Keywords: GM1, Spatial Learning, NBM, Ganglioside, Y maze
Cite this paper: Sedigheh Ashkavandi , Ahmad Ali Moazedi , Saeed Semnanian , Hooman Eshagh Harooni , Mahdi Pourmehdi , Effects of GM1 Ganglioside on the Recovery of Spatial Learning after a Lesion of the Nucleus Basalis Magnocellularis in an Experimental Model of Alzheimer’s Disease in Adult Male Rats, American Journal of Medicine and Medical Sciences, Vol. 5 No. 1, 2015, pp. 42-47. doi: 10.5923/j.ajmms.20150501.08.
1. Introduction
- Alzheimer’s disease (AD) is the most frequent cause of cognitive decline and dementia, affecting more than 36 million people worldwide. The principal pathologic features found in postmortem brains of AD sufferers are extracellular amyloid plaques, formed by aggregated amyloid-beta (Aβ) peptides, and neurofibrillary tangles composed of paired filaments of abnormally phosphorylated tau protein [1, 2]. Researchers have found that decreases in size and in the density of neurons within the basal forebrain, particularly in the NBM, are aspects of the neuropathology of AD. The NBM, one of the nuclei of the basal forebrain, provides the major cholinergic innervation to the frontal, prefrontal and parietal areas of the cerebral cortex. The NBM also sends substantial cholinergic projections to the amygdala. Several investigators have reported that damage to the NBM significantly reduces cortical acetylcholine levels and that decreases in cortical acetylcholine levels are associated with cognitive impairments in both humans and animals [3]. Thus, lesions to the NBM in animals have been used in an attempt to understand the cognitive deterioration seen in AD. The NBM in rats is equivalent to the nucleus basalis of Meynert (nBM) in humans [4]. The electrical lesion of NBM is used to mimic the Alzheimer’s features in rodents [5].GM1 ganglioside is a sialic acid containing the glycosphingolipid, found in the plasma membrane of most vertebrate cells, particularly in the nervous system [6]. Gangliosides a type of glycolipids have been implicated in many cellular functions including cell to cell recognition, interactions with extracellular proteins, receptor for hormones, and viruses such as influenza and Sendai, and receptors for toxins such as cholera, tetanus, and botulinum [7]. Also, in the central nervous systems, gangliosides are involved in many physiological functions such as neurogenesis, proliferation, synaptogenesis and synaptic transmission [8]. The results suggest that in hippocampal CA1 neurons, gangliosids participate in activity-dependent LTP through the modulation of NMDA receptors/ca2+ channels activity [8, 9]. Some gangliosides induce neurogenesis and exhibit a trophic effect on nerve cells grown in vitro. In vivo, a particular ganglioside, GM1, reduces cerebral edema and accelerates recovery from injury (traumatic and ischemic) to the peripheral and central nervous systems of laboratory animals. Preliminary clinical studies have shown that treatment with gangliosides may have corresponding effects on lesions of the human peripheral nervous system [10]. In the present study, the effects of GM1 on the spatial learning and memory in experience Alzheimer’s model in adult male rats have been investigated.
2. Materials and Methods
- Thirty-five male rats, obtained from animal house of Ahwaz Jondishapur University of Medical Sciences (AJUMS), at the beginning of the experiment. All rats were housed foursome and kept under conditions of controlled temperature (20-23℃) and humidity (40-70%) and to a light/darkness cycle of 12/12 h (lights on at 7:00 am). Food and water were available ad libitum.Animals were randomly allocated into five groups: Control group (educated in Y-maze without any administration), Lesion group were received lesion with electrically method: 0.5mA, 3s (bilaterally lesion of NBM to create Alzheimer’s model), Sham group (entering the electrode to the NBM without any currents), GM1 group (lesion+ 2 mg/kg GM1) and saline group (lesion NBM + 0.2 ml saline). Stereotaxic surgery: In order to create animal model of Alzheimer’s disease. The NBM of animals was destroyed bilaterally with electrical lesion (0.5 mA for 3 sec) while under anesthesia induced by injection of Ketamine (78 mg/kg, i.p., Alphasan, Holland) and Xylazine (3mg/kg i.p. Alphasan, Holland) and stereotaxic surgery (AP = -1.3mm, ML = ± 2.8mm, DV = -7.6mm) [11]. Y-maze task: The maze was made of black painted Plexiglas. Each arm was 60 cm long, 30 cm high and 17.5 cm wide. The arm converged in an equilateral triangular central area that was 17.5 cm at its longest axis. Two days before the beginning of each experimental session, the rats were transported in its living cage from the colony cage to the testing room. Y-maze apparatus was put in a completely dark and silent place. To knowing the apparatus, at the first day of the training, rats were allowed to have a freely moving for 15 minutes. Then training was begin from the arm that rat was there. Each rat was trained 30 trials every day and training was continued for 5 days. At the end of each session, Correct Response Percentages (CRP) was calculated. Minimum CRP was considered as 86.6% [12]. One month later, spatial memory performance was assessed by recording spontaneous alternation behavior in a single session in Y-maze.Statistical analyses: Data were analyzed with SPSS software using One Way ANOVA procedure, then for determining the significant difference between groups, Least Significant Difference (LSD) procedure were used. The minimally acceptable level was set as p<0.05.
3. Results
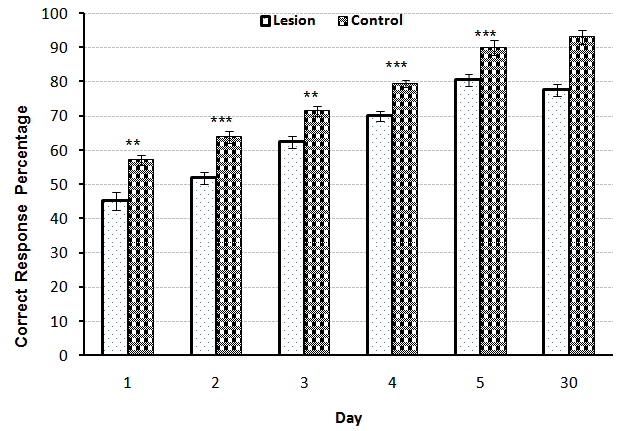
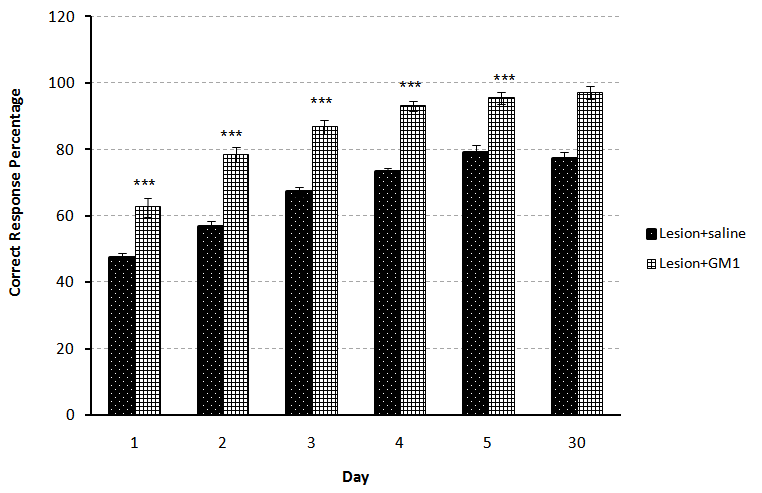
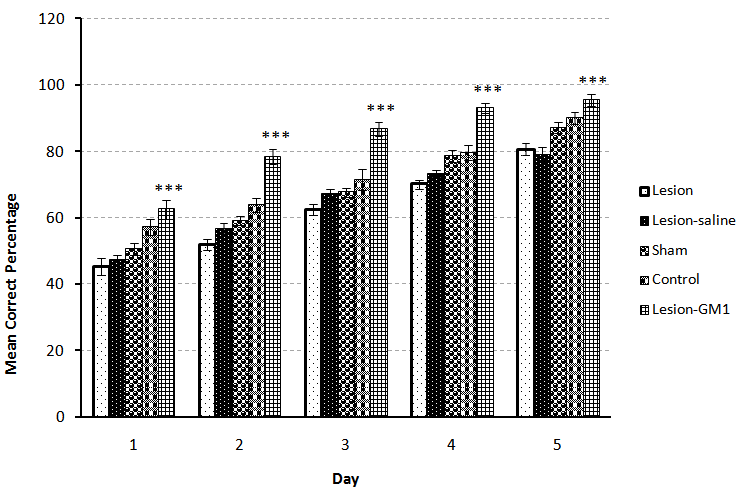
- A significant difference observed between control (n=7) and Lesion (n=7) groups at the first and third (p<0.01), second, fourth and fifth (p<0.001) days of training, it means that lesion of NBM caused reduction of spatial learning (Fig.1). Also comparison between Control (n=7) and Sham (n=7) groups showed that there was no significant difference, revealed that entering electrode without induction of current, had no effect on spatial learning (Fig.2). On the other hand, comparison between Lesion+ GM1 (n=8) and Lesion+ Saline (n=7) groups showed a significant difference (p<0.001) in every five days of training. Also ANOVA between all groups showed that there was a significant difference between Lesion+GM1 and other groups (p<0.001) (Fig 4.), demonstrate that GM1 improve the spatial learning in experimental model of Alzheimer’s disease, nevertheless Saline has no effect on the spatial learning. There were no differences between the comparison of CRP in fifth day of training and the one month after’s memory test in all groups.
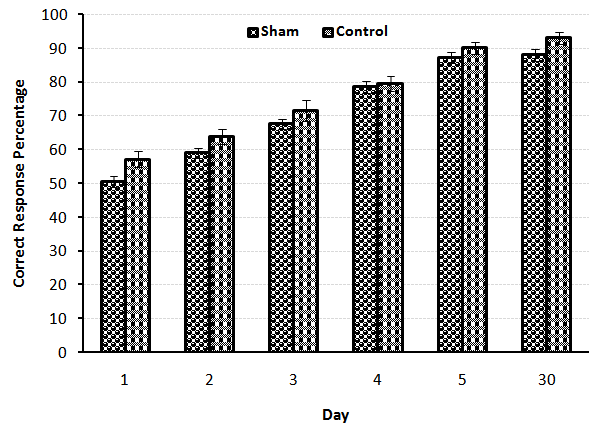 | Figure 2. Comparison of Correct Response Percentage (X¯± S.E.M) between the Control and Sham groups during five days training and the memory test in adult male rats(n=7) |
4. Discussion
- The severity of dementia in AD depends on the loss of neurons in the NBM with large cells and significant decrease in the amount of acetylcholine transferase enzyme in brain cortex and amygdala results in impaired learning and memory [13]. Our results have shown that the bilateral lesions of NBM by electric current in rats has induced significant learning disturbance in spatial learning by Y-maze task comparing with the control group (p<0.001) (Fig. 1). There are some studies found that the NBM lesion electrically or chemically, induced disturbances in spatial cognitive [11], acetylcholine transferase levels on the cortex and hippocampus [3] and the olfactory discrimination learning set formation [14]. Also comparison between control and sham groups indicated that there were no significant differences between these groups, suggested that different stages of surgery including anesthesia and entering electrode without inducing any current, had no effect on spatial learning in the animals (Fig.2).In addition, the comparison between Lesion+ GM1 and Lesion+ Saline groups indicated that GM1 increased spatial learning significantly in Alzheimer’s experience adult male rats, but saline had no effect on the spatial learning (Fig. 3). There are many studies found that GM1 has a positive effect on the learning [15-18], choline acetyltransferase (ChAT) and acetyl cholinesterase recovery after CNS injury [19-22]. The possibly mechanisms for the beneficial effects of GM1 and other gangliosides are reporting following:1- GM1 is an important modulator of calcium efflux across the nuclear membrane during neuronal development. It is demonstrated that because of the tight connections between nuclear and endoplasmic reticulum membranes, GM1 could be complicated in the maintenance of calcium homeostasis in the ER compartment [22].2- Using hippocampal slices, the protection of Na+, K+- ATPase activity by either in vitro or in vivo administration of GM1 ganglioside has been reported. Exogenous gangliosides have been shown to functionally insert into membranes and to modulate ATPase activity in vitro and in vivo [23].3- GM1 treatments protect neuronal as well as neuromuscular resistance to hypoxia and ionic disturbances, improve conduction velocity and axonal morphology in neuropathy, prevent ultra-structural deterioration of mitochondria, and reduce retrograde axonal degeneration in the CNS after injury. These data, together with evidence for gangliosides' ability to reduce cerebral edema and mortality rates due to significant CNS injury, strengthen the hypothesis that gangliosides may preserve membrane structure by preventing the "deterioration of the membrane microenvironment” [24].4- Tyrosine kinases of trophic factor receptors are sites for the action for the gangliosides including GM1 [25]. 5- Gangliosides might enhance the function of cholinergic neurons and the initial clues of that, came from observations that, in cats with GM1 gangliosidosis, there is a marked increase of acetylcholine synthesis, K+-stimulated acetylcholine release [26], and high-affinity choline transport (HAChT) activity in the cortex and hippocampus [26, 27]. It is interesting that several polysialogangliosides appear to be selectively localized to cholinergic neurons [28-31]. 6- Finally, GM1 prevents glutamate receptor-mediated neurotoxicity [32].On the other hands, there are some studies inconsistent with our results found that GM1 metabolism in Alzheimer’s patients has enhanced [33, 34], GM1 ganglioside reduces cognitive dysfunction after focal cortical ischemia [35]. GM1 reduced in lesion-induced place learning deficits on the task of Morris water maze [36], Meanwhile GM1 up-regulates ubiquilin 1 expression (one of the candidate genes of AD) in human neuroblastoma cells and rat cortical neurons [6]. So according to the obtained results, it is possible that GM1 by using some mechanisms such as: enhancing the calcium efflux, improving the function of cholinergic neurons or protection of Na+, K+- ATPase activity, improved the spatial learning in the experimental model of Alzheimer’s disease in adult male rats.
ACKNOWLEDGMENTS
- All experiments were performed in the Laboratory of Learning and Memory in Biology Department of Shahid Chamran Inuversity in Iran.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML