| [1] | Al-Allaf LI, Taher MT, Al-Mukhtar SBA, Al-Dulaimy LH: Histochemical study of alkaline phosphatase enzyme in gallbladder containing cholesterol stones. Ann Coll Med Mosul 2013, 39 (2):166-171. |
| [2] | Al-Obaidi SMM, Abdulla TS, Al-Alawi MS, Mohammed HMK: The prevalence of silent gall stones and Its relation to some risk factors in Iraq, The Iraqi postgraduate medical Journal 2006,5(2):146-150. |
| [3] | Atamer A, Övünc AOK, Yeşil AY, Atamar Y: Evalution of leptin and insulin resistance in patients with cholelithiasis, Indian Journal of biochemistry and biophysics 2013,50(4): 266-272. |
| [4] | Belousov Y. V. Pediatric Gastroenterology. Up-to-date guide. Moscow: Exama, 2006, p. 112. |
| [5] | Channa NA, Khand F, Ghanghro AB, Soomro AM: Quantitative analysis of Serum Lipid profile in Gallstone patients and Controls, Pakistan J Anal Environ Chem 2010, 11(1) 59. |
| [6] | Channa NA, Shaikh HR, Khand FD, Bhanger MI, Laghari MH: Association of gallstone daises risk with serum level of alkaline phosphatase, JLUMHS 2005, 4(1):18-22. |
| [7] | Chen LI, Qiao QH, Zhang SC, Chen YH, Chao GQ, Fang LZ: Metabolic syndrome and gallstone disease. World J Gastroenterol 2012, 18(31): 4215-4220. |
| [8] | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. ClinChem 1972, 18(6):499-502. |
| [9] | Kim SS, Lee JG, Kim DW, Kim BH, Jeon YK, Kim MR, Huh JE, Mok JY, Kim SJ, Kim YK, Kim IJ: Insulin resistance as a risk factor for gallbladder stone formation in Korean postmenopausal women, Korean J Intern Med 2011, 26(3): 285–293. |
| [10] | Kumari DJ, Krishna SHB: Role of body mass index, physical activity and nutrients in cholelithiasis in guntur, Andhra Pradesh. J Hum Ecol 2010, 31(3): 151-155. |
| [11] | Marschall HU, Einarsson C: Gallstone disease. J Inter Med 2007, 261:529-542. |
| [12] | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28(7): 412-419. |
| [13] | Mendhez-Sanchez N, Ponciano-Rodrigoez G, Chavez –Tapia N, Uribe M: Effects of leptin on biliary lipids: potential consequences for gallstone formation and therapy in obesity. CurrDrug Targets Immune Endocrmetabol disorder 2005, 5(2): 203-208. |
| [14] | Misciagna G, Vguerra A, Leo D, Correale M, Trevisan M: Insulin and gallstone: apopulation case study in southern Italy.Gut2000, 47:144-147. |
| [15] | Reaven GM: Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med 2005, 47(4):201-210. |
| [16] | Reshetnyak, V.I. Concept of the pathogenesis and treatment of cholelithiasis, World J Hepatology 2012, 27; 4(2): 18-34. |
| [17] | Ruhl C. E. and Everhart JE: Relationship of serum leptin concentration and other measures of adiposity with gallbladder disease. Hepatology 2001, 34(5): 877-883. |
| [18] | Sharma R, Sachan SG, Sharma SR: Preponderance of gallstone in female. World Journal of Pharmacy and Pharmaceutical Sciences 2013, 2(6): 5871-5877. |
| [19] | Sharma R., Kumar A, Jha NK, Sacchan SG, Vidyarthi AS, Sharma SR: Probing into the prevalence and factors of gallstone formation in Jharkhand region in India, International journal of applied biology and pharmaceutical technology. 2012, 3(3):36-41. |
| [20] | Sun H, TangH, Jiang S, ZengL, ChenEQ, ZhouTY, Wang YJ: Gender and metabolic differences of gallstone diseases. World J Gastroenterol 2009, 15(15): 1886–1891. |
| [21] | Taher MA: Descriptive study of cholelithiasis with chemical constituents’ analysis of gallstones from patients living in Baghdad, Iraq, Int J Med Medical Sci2013, 5(1):19-23. |
| [22] | Tassaduqe K, Ali M, Salam A, Latif M, Afroze N, Masood S, Soban U: Studies on the chemical composition and presentation of gallstone in relation to sex and age among human population of Multan, Pakistan. J Bio Sci2004, 4(4): 470-473. |
| [23] | Tirziui S, Bel S, Bondor CI, Acalovschi M: Risk Factors for gallstone disease in patients with gallstones having gallstone heredity. A case-control study. Rom J InterMed 2008, 46 (3): 223–228. |
| [24] | Wittenburg H: Hereditary liver disease: Gallstones. Best Practice & Research Clinical Gastroenterology2010, 24:747–756. |

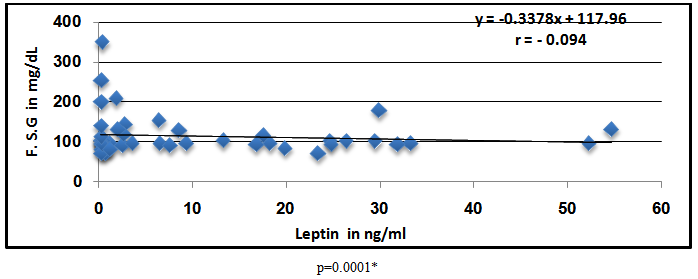
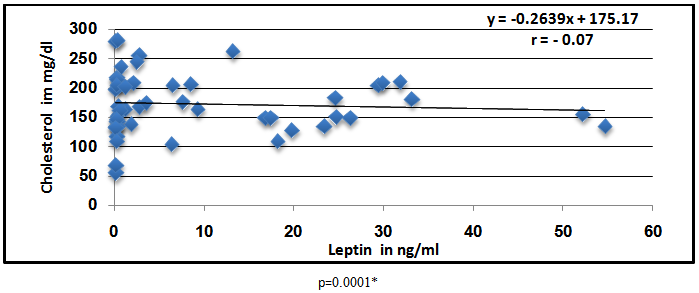
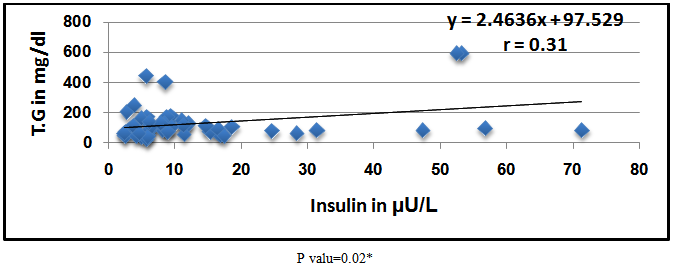
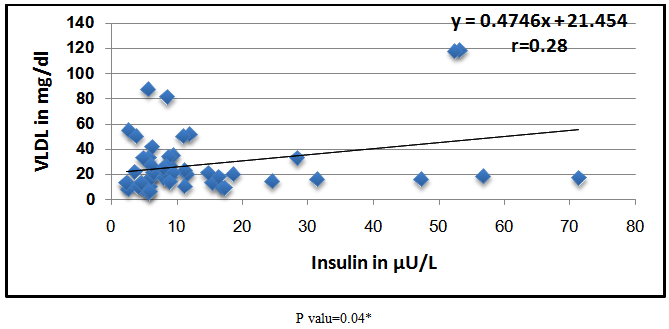
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML