-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(1): 15-19
doi:10.5923/j.ajmms.20150501.04
Interleukins IL-6, IL8, IL10 and Tumor Necrosis Factor TNF Expression in Human Infected with Giardiaduodenalis
Najwa Shihab Ahmed1, Fadia Abd Al-Muhsin AL-Khayat2, Farah Thamer Abdullah1
1Biotechnology Research Center, Al-Nahrain University, Baghdad, Iraq
2Dep. Basic Sciences, Dentistry College, Baghdad University, Baghdad, Iraq
Correspondence to: Najwa Shihab Ahmed, Biotechnology Research Center, Al-Nahrain University, Baghdad, Iraq.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Giardia duodenalis is the only species within the Giardia genus that infects humans, pets and livestock and is a common cause of both acute and chronic diarrheal disease worldwide. Both humoral and cell-mediated immune responses play a role in acquired immunity. The purpose of this study was to determine the prevalence of G.duodenalis and the levels of Interleukins IL-6, IL-8, IL-10 and Tumor Necrosis Factor (TNF) in patients suffering giardiasis. During the period of the study (June, July and August / 2014), a total of 255 human stool samples (aged 15-35) were examined under microscope for the detection of the causative agent by using saline wet mount method, blood samples (3ml) were collected from infected patients to determine the production of IL-6, IL-8, IL-10 and TNF in serum compared to uninfected persons (20 sample) by using Enzyme link Immune Sorbent Assay (ELISA). The overall infection rate of G.duodenaliswas 23.52%, 60 person were infected from 255, according to genderhigher infection rate was recorded in males 25% (44 from176) compared to the females rate 20.25% (16/79) no significant differences were observed between infection rates (Chi-square=0.68, P value=0.44). A significant difference (p˂0.05) was recorded in the mean concentrations of IL-6 and TNF between infected (1.97 pm/ml, 0.171 pm/ml) and uninfected (0.272pm/ml, 0.061pm/ml) respectively, while no significant differences were observed in the mean concentration of IL-8 and IL-10 between serum examined samples of infected (0.126pm/ml, 0.054pm/ml) and uninfected persons (0.106pm/ml, 0.059pm/ml). The immune system response to G.duodenalisinfection was detected through expression of interleukins such as IL-6 and TNF.
Keywords: Giardiasis, Prevalence rate, Immunity cytokines production
Cite this paper: Najwa Shihab Ahmed, Fadia Abd Al-Muhsin AL-Khayat, Farah Thamer Abdullah, Interleukins IL-6, IL8, IL10 and Tumor Necrosis Factor TNF Expression in Human Infected with Giardiaduodenalis, American Journal of Medicine and Medical Sciences, Vol. 5 No. 1, 2015, pp. 15-19. doi: 10.5923/j.ajmms.20150501.04.
Article Outline
1. Introduction
- Giardia duodenalis (synonymous with G.lamblia, G.intestinalis) is a worldwide parasitic protozoan which caused gastrointestinal infection called giardiasis which has been targeted as part of the WHO “Neglected Disease Initiative” since 2006 (Savioli, et al., 2006), with a broad range of clinical manifestations from asymptomatic carriage, chronic diarrhea to severe malabsorption. The symptoms of Giardia infection can vary in intensity from person to person, many people may have no symptoms at all while, others showed symptoms of diarrhea that lasts ten days or more, abdominal pain, nausea, vomiting, fever, chills, bloating, gas, and weight loss. Complications include severe dehydrationdue to the loss of fluids and electrolytes which can lead to an electrolyte imbalance and shock, and can even be fatal (Nash and Patel, 2010). Differences in clinicalmanifestations may be due to a number of factors, including host age, immune and nutritional status, concurrent infections, the virulence and pathogenicity of the Giardia strain (Aliand Hill, 2003). Transmission of Giardiais via the fecal-oral route, either directly from person-to-person or animal-to-person contact or indirectly through contaminated water or food (Barry, et al., 2013).The defense against infection is acting locally because G.duodenalis was not invade the epithelial layer and infection is typically characterized by little or no mucosal inflammation (Li, 2004). Potent immune response is important for eradication of the parasite during infection and development of protective immunity, both cellular and humoral mechanisms are important in the resistance to giardiasis (Fanbert, 2000). Cytokinesor Interleukins may have a role in G.duodenalis infection. Interleukins are secreted by lymphocytes, monocytes or macrophages. They act on other cells of the immune system to regulate their function. Interleukins cause inflammatory response in parasitic diseases. The present work was undertaken to determine the total infection rate and the levels of interleukins (IL-6, IL-8, IL10 and TNF) in the sera of infected persons.
2. Material and Methods
- A total of 255 human stool samples were collected from patients (about 5 g in sterile plastic cups) during the period of three months June, July and August / 2014fromAL-Yarmouk hospital in Baghdad. Samples (included 176 male and 79 female aged from 15 - 35 year) were examined under microscope (40Xlens) by using saline wet-mount method (1g of stool in 1 ml of normal saline) for G.duodenalis detection (Alam, et al., 2011). Serum interleukins (IL-6, IL-8, IL-10 and TNF) were estimated in 60 patients suffering from giardiasis and 20 apparently healthy controls (10 for each male and female), interleukins were determined by ELISA. Samples of blood were collected (3ml) in plane tube from patients, serum was separated by centrifuged the blood at 3000 rpm for 5 minutes and stored in Eppendorf tube at -20 until use.
2.1. Detection of Interleukin (IL-6, IL-8) by ELISA Method
- Following the instructions ofIL-6 Cytokine Kit, catalog number HS600B and the IL-8 Cytokine Kit, catalog number D8000C, frozen serum samples were brought to room temperature and mixed thoroughly without foaming. One hundred μL of Assay Diluent (RD6-11 for IL-6 and RD1-85 for IL-8) was added to each well, 100 μL of control and sample were added to each per well. The Covered plate with a fresh sealer was incubated for 2h at room temperature 25ºC and shaker at 500 ± 50 rpm. The plate was washed by dispensed 0.4ml of washing solution into each well then the content of well was aspirated (washing was repeated twice). 200 μL of interleukin Conjugate was added to each well. The Covered plate was incubated for 2h at room temperature 25ºC and shaker at 500 ± 50 rpm then washed as in up step, 50 μL of substrate solution was added to each well, the covered plate was incubated for 1h at room temperature 25ºC and shaker at 500 ± 50 rpm. Then 50 μL of Amplifier Solution was added to each well, incubated for 30 minutes at room temperature. Then stop solution was added (50μL) to each well, the absorbance of each well was recorded by using ELISA microplate reader (Olympus/ Japan) at 490nm.
2.2. Detection of Interleukin IL-10by ELISA Method
- Practical work was done following the instructions of US Biological IL-10 kit protocol/Biochemical &Biological Reagents, United State Biological, catalog No (18432-05). One hundred microliter from each standard and samples were added (in duplicate) into the antibody pre-coated microtiter plate, then incubated for 1 hour at room temperature. Without discarding standards or samples, solutions about 50 µl Pab (biotin) was added to each well, incubated for 1 hour at room temperature then the plate was washed to remove any unconjucated antibodies. The Avidin attached with HRP enzyme was added to all wells in quantity of 100 µl, the plate was incubated in dark at room temperature for another 1 hour followed by washing step, finally 100 µl substrate mixture was added for 15 minutes stand period in dark at room temperature then to stop their reaction, 100 µl stop solution was added. At the end of experiment a standard curve for different standard concentrations verses their absorbance at 620nm were plotted, then each IL-10 concentration was calculated and then evaluated statically.
2.3. Detection of Tumor Necrosis Factor (TNF) by ELISA
- The kit depend on TNF-α enzyme linked immune sorbent assay (ELISA), which is a quantitative sandwich immunoassay allows good determination for concentration of TNF-α in the sample, following the instruction of US Biological TNF-α kit protocol/Biochemical & Biological Reagents, United State Biological. Catalog No (T9160-01). The microtiter plate provided in this kit has been pre-coated with a monoclonal antibody specific to TNF-α. Controls or samples were then added to the appropriate microtiter plate wells with a biotin-conjugated polyclonal antibody preparation specific for TNF-α, if present, the TNF-α will bind and become immobilized by the antibody pre-coated on the wells and then be "sandwiched" by biotin conjugate. In order to measure the concentration of TNF- α in the samples, this kit includes two calibration diluents. According to the testing system, the provided standard was diluted (2-fold) with the appropriate Calibrator Diluents and assayed at the same time as the samples. This allows the operator to produce a standard curve of Optical Density (O.D) versus TNF- α concentration (pg/ml). The concentration of TNF- α in the samples was then determined by standard curve and the straight line equation.Statistical analysis was performed using SAS (2012). Means were compared by unpaired T-test to assess significant differences. Proportions were compared by chi-square. P < 0.05 was considered statistically significant
3. Results
3.1. Infection rate
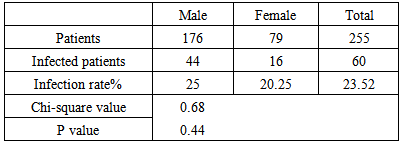
- The results of the study revealed that 60 patients from 255 showed G.duodenalis in stool samples (cyst, trophozoit), the total infection rate recorded 23.52%. The positive samples included 44 male and 16 female with infection rates recorded 25%, 20.25% respectively. Difference between infection rates was non-significant. (Table 1, Figure 1).
|
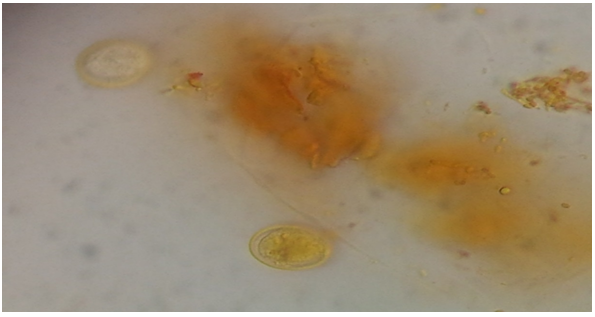 | Figure 1. Giardiaduodenalis cyst in human stool sample |
3.2. Detection of Interleukins by ELISA
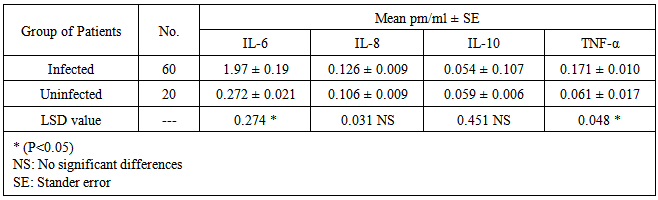
- The levels of Interleukins 6,8,10 and tumor necrosis factor TNF-α were detected using ELISA method in 60 serum samples of infected patients with another 20 serum samples from uninfected, Concentration was calculated using the equation Specials:IL-6(y=0.205x+0.571), IL-8(y=0.185x+0.225), IL-10(y=0.003x+0.001) and TNF-α (Y=0.0024x + 0.0431).The results showed significant differences in the mean concentrations between infected and uninfected persons for IL-6 and TNF while no significant differences were recorded in IL-8 and IL-10. (Table 2).
|
4. Discussion
- The immune system has multiple weapons which it uses to help control infections. Many infections result in activation of several of these response mechanisms, but it is not always clear which responses actually contribute to control of the pathogen and which are bystander effects. Data from humans suggest that antibody responses are important in preventing chronic infections, although roles for cellular responses have not been excluded (Solaymani-Mohammadi and Singer, 2010). Interleukins also called cytokines are hormone like polypeptides produced by lymphocytes, monocytes or macrophage and consist of lymphokines and Interleukins are released in response to inflammatory stimuli and act on other cells of the immune system to regulate their function (Ncta, et al., 1988).In giardiasis, most of the interleukins are produced by CD4 + of Peyer's patches or generated from the mucosa associated lymphoid tissue as a result of long duration antigenic stimulation via or cystic stage of G.duodenalis (Scott, et al., 2004). Type and amount of these cytokines responses may be affected by the infecting parasite whether it is invasive or non-invasive (Jung, et al., 1995).In the present study,IL-6 was increased in infected persons and the mean concentration recorded significant differences between infected and uninfected persons, this result showed similarity to that proved by Zhou, et al (2003) in which elevated IL-6 expression was found in wild-type mice 15 days post infection and concluded that IL-6 is necessary for early control of acute Giardia infections.IL-6 stimulates B cell differentiation and antibody produced, T-cell proliferation, and modulates the IgA response.The results showed no increase in IL-8 and IL-10 concentrations which indicate the minor role of Th2 cytokines in immune response to giardiasis, IL-8 is the major chemokine responsible for neutrophil recruitment to sites of tissue insult and inflammation. Production of IL-8 by monocytes and neutrophils stimulated by live trophozoites (Mukaida, 2000). This finding was agree with the results reported in a study showed that these cytokines may have no influence on immunity to giardiasis and explain the chronic nature of this disease as G.duodenalis normally does not penetrate the epithelial barrier therefore the spontaneous elimination of the parasite depends largely on immune mechanism. Giardia cause chronic diarrhea and malabsorption and the T-cell activation and cytokines release associated with mucosal inflammatory produced (Farthing, 1993; Venkatesan, et al., 1996). Jung, et al (1995) reported that IL-8 was not induced in Giardia infection which explains the low levels of inflammation in contrast with bacterial infection. In another study, similar results revealed that neither IL-10 nor IL-12 were secreted and only small quantities of IL-6 and TNF-α were produced. Interestingly, IL-6 and TNF-α are the only two cytokines among several tested thus far that have been shown to be necessary for effective Giardia control in mice (Joe and Steven, 2009). Also Cotton, et al. (2014) showed that G. duodenalis trophozoites attenuate secretion of the potent neutrophil chemoattractant interleukin-8.TNF-α was elevated in infected persons with the mean concentration recorded 0.171 Pm/ml this may be due to that TNF-α is a mediator of inflammatory response also plays an important role in parasitic diseases (Clark, et al., 1987). TNF-α, a cytokine produced by macrophage is a portend mediator of inflammatory and immunological reaction (Beutler and Cerami, 1989). Bienz, et al. (2003) reported that the two cytokines which have been shown to play an important role in control of giardiasis are IL-6 and TNF-α. The result is in agreement with a study included a total of 126 subjects; 52 giardiasic children were detected and TNF-α levels significantly increased in these cases. (Bayraktar, et al., 2005). Another conclusion reported by Samira, et al. (2010) showed similarity, in which parasite derived antigen increased TNF-alpha and enhanced production of IL-6.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML