-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(1): 10-14
doi:10.5923/j.ajmms.20150501.03
Immunologic Study in Mice Immunized with Whole Cryptococcus Neoformans Fungusorganism
Tareq Jaafar Aljindeel1, Shiama Nabhan Al-Deliamy2, Aseel Ibrahim Al-Ameed2
1College of Vet. Med., Al-Muthana University, Iraq
2Bagdad University
Correspondence to: Tareq Jaafar Aljindeel, College of Vet. Med., Al-Muthana University, Iraq.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
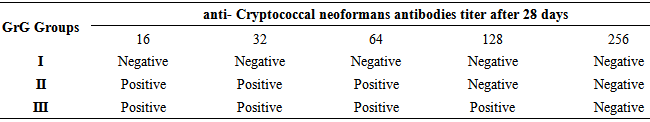
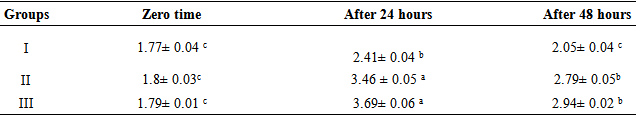
A comparative study was conducted to focus on immune response level in different groups assist by some immunologic parameters in mice immunized with the whole cell killed yeast Cryptococcus neoformansvaccine. All experiments were carried out on a total number of120 male and female albino mice (Blab-c), mice were divided into equal three groups, the first group received distilled water as (control group), the second group was immunized with killed yeast Cryptococcus neoformans antigen and the third group was immunized with a life yeast Cryptococcus neoformans antigen. Vaccine efficiency was evaluated according to phagocytic activity percentage, delayed type hypersensitivity reaction test, anti-C.neoformans antibodies titer level and gamma globulin fraction percentage in immunized mice serum. All treatments were carried out on day one. Then the mice were scarified and tested at different periods: day 10, phagocytic activity percentage by Nitro Blue Tetrazolium test (NBT) was evaluated, at days 14 tests for delayed hypersensitivity type reaction of skin, and at day 21 and 28 performed the test for anti-anti-C.neoformans antibodies titerlevel in mice serum by indirect immunoflourescent assay and gamma globulin fraction percentage by Gel electrophoresis. Results revealed that third group recorded significantly higher values in their peripheral blood phagocytic activity by nitro blue tetrazolium test measured by ELISA reader and anti- C.neoformansantibodies titer level in mice serum at 21 and 28 days by indirect immunoflourescent assay and gamma globulin fraction as compared with control group. Second group results revealed significantly increased in phagocytic activity index of peripheral blood by nitro blue tetrazolium test and the anti- C.neoformans antibodies titer level assessed by indirect immunoflourescent test and gamma globulin fraction in mice serum at 21 and 28 days. In delayed-type hypersensitivity reaction, the index was significantly increased in group III vaccinated mice and in group II as comparison with control group, the best results was observed after 24 hours post-C.neoformansprotein injection. Result concluded that a life C.neoformans antigen produce the best immune response in mice.
Keywords: a life and heat killed C.neoformansimmunization in mice
Cite this paper: Tareq Jaafar Aljindeel, Shiama Nabhan Al-Deliamy, Aseel Ibrahim Al-Ameed, Immunologic Study in Mice Immunized with Whole Cryptococcus Neoformans Fungusorganism, American Journal of Medicine and Medical Sciences, Vol. 5 No. 1, 2015, pp. 10-14. doi: 10.5923/j.ajmms.20150501.03.
1. Introduction
- Cryptococcus neoformans is an opportunistic encapsulated fungal pathogen that causes significant morbidity and mortality in immunocompromised individuals, including patients with AIDS and other immune defects [1]. Cryptococcus neoformans is a yeast-like fungus which causes life-threatening meningoencephalitis in 5 to 10% of patients with AIDS. Cryptococcosis is still a significant problem in Asia and Africa [1]. The infectious particle enters the host through the respiratory tract and reaches the lung, where the primary infection is contained in immunocompetent hosts. In contrast, the ability to control infection is severely hampered in immuno-suppressed subjects, resulting in dissemination of fungal cells to cause life threatening meningitis [2]. However, cryptococcosis is an emerging problem in other immunocompromised patient populations and remains a major cause of meningoencephalitis in the developing world [1, 3]. Therapeutic optionsare inadequate to eradicate the fungus. It is widely recognized that a complex interplay between cell-mediated and humoral immune response plays a pivotal role in the control of C. neoformans infection [4]. Despite the availability of antifungalagents that are active against C. neoformans, cryptococcosis is largely incurable in individuals with immune impairment because the organism cannot be completely eradicated [5]. Hence, modalities that enhance or provide components of the protective immune response to C. neoformans represent a rational approach to the management of cryptococcosis [6, 7]. As such, immune-based adjunctive antibody-based therapiesare promising modalities because of their ability to augment host defense mechanisms against C. neoformans [6, 8]. In animal models of infection, there is convincing evidence that administration of preformed antibody to the polysaccharide capsule can prolong survival and reduce organ tissue fungal burden [6]. The efficacy of some antibodies against C. neoformans has led to the development of a highly immunogenic polysaccharide-protein conjugate vaccine for the prevention of cryptococcal infection [9]. Cell-mediated immunity has been extensively implicated as an important defense mechanism against C. neoformans infection [1].
2. Material and Method
- ACryptococcus neoformans isolate from poultry worker suffering from respiratory infection. Two strains of C. neoformans var. neoformans were used: a serotype A thinly encapsulated strain (CBS 6995 5 NIH 37; National Institutes of Health, Bethesda, Md.) and a capsular mutant (CBS 7698 5 NIH.All experiments were carried out on male and female albino mice (Blab-c), which were supplied by the National Centre for Drug Control and Research Baghdad/Iraq. The starting age of mice is rounded eight weeks. They were housed in bio-clean hoods at 20-25℃ with light, dark periods of 14:10 hours. They were fed standard pellets and water, their initial weight was 25 ± 3 grams at the beginning of Experiments. Mice were separately caged for a one week preliminary period for acclimatization period. The following culture media were used in carrying out the experiments of the study (Blood agar, Sabourauds dextrose agar, Trypticase Soya agar, Trypticase Soya broth and yeast extract) was the products of Difco Company (U.S.A). The life yeast Cryptococcus neoformansvaccine prepared as described by [1] and killed yeast vaccine prepared as described by [1]. The NBT kit used in the study was the product of Sigma company (U.S.A). There were three groups in this experiment, which was designed to evaluate the immune response in mice vaccinated with Cryptococcus neoformans vaccine. 120 mice were a total number, 40mice in each group). All mice were treated on the day [1] subcutaneously:Group I: A mice were injected subcutaneously with a single dose (0.2ml) of deionized distilled water in cervical region atday 1. Group II: mice were vaccinated with 0.2 ml of killed yeast Cryptococcus neoformans vaccine in a similar manner. Group III: mice were vaccinated with 0.2 ml of a life Yeast Cryptococcus neoformans vaccine in a similar manner; all above treatment were done at day 1. All mice were sacrificed on the day 8 to evaluated (phagocytic activity by NBT index measured by Elisa reader as suggested by [10], on the day 14 to evaluate (delayed-type hypersensitivity reaction) as suggested by [3] and in day 21 and 28 to assess anti-Cryptococcal neoformans antibody titer by indirect Fluorescent test in the sera of mice that were immunized with a life and killed Cryptococcus neoformans vaccine, the procedure of WHO 1997 [11] was adopted to determine such titer. Serum electrophoresis was carried out using a commercially available kit (Hellabio, Spain) for evaluated of gamma globulin fraction in immunized mice. The Hellabio Agarose Gels for protein electrophoresis are intended to be used for in vitro diagnosis, and they enable quantitative and qualitative estimation of proteins in serum and other biological materials.
3. Result and Dissection
- The results of NBT index were given in table 1. Mice in groups II and III showed different significant increases in the NBT index test which represented the phagocytic activity% (65.99% and 77.45% respectively) as compared to group I (0%), which was injected with deionized distilled water (control group). The best NBT index was recorded in group III.
|
|
|
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML