-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2015; 5(1): 1-6
doi:10.5923/j.ajmms.20150501.01
High Sensitive C-Reactive Protein(hs-CRP) as Predictor Marker for Cardiovascular Disease among Vitamin D Deficient Hypertensive Patients
Rawia Abdalla Mohamed 1, Muzamil Mohammed Musa 1, Amar Mohamed Ismail 2
1Department of Clinical Chemistry, College of Medical Laboratory Science, Sudan University of Science & Technology, Sudan
2Department of Biochemistry & Molecular Biology, Faculty of Science & Technology, AL-Neelain University, Sudan
Correspondence to: Amar Mohamed Ismail , Department of Biochemistry & Molecular Biology, Faculty of Science & Technology, AL-Neelain University, Sudan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Background: High sensitive C-reactive protein (hs-CRP) is acute-phase protein which described as sensitive systemic marker of inflammation and tissue damage especially in cardiovascular disease (CVD). Vitamin D may reduce hypertension prevalence and its complication since vitamin D deficiency is associated with CVD, therefore this study aims to evaluate hs-CRP as a predictor marker for CVD in vitamin D deficient hypertensive patients. Materials and Methods: In cross-sectional study 176 hypertensive patients were enrolled (92 male, 84 female), then classified based on vitamin D results into normal (>20 ng/ml) and case (<20 ng/ml) groups. Serum vitamin D level was estimated using competitive ELISA assay and serum hs-CRP level was measured by Cobas C-311 automated analyzer, TG, HDL-C and total cholesterol were measured using enzymatic spectrophotometric methods whereas LDL-C and LDL:HDL ratio were calculated. Results: hs-CRP was significantly increased in vitamin D deficient compared to control group with p-value 0.049, also significantly higher in overweight hypertensive patient’s p-value 0.014. Vitamin D deficient was observed in female, overweight and >5 years duration of hypertension subjects in comparison with male, normal weight and <5 years with p-values (0.000, 0.005 and 0.041) respectively. Cholesterol was significantly higher in overweight group p-values 0.045, while LDL level was significantly higher and HDL was significantly lower in >5 years duration of hypertension. Conclusions: The study concludes hs-CRP is useful predictor marker for cardiovascular disease in vitamin D deficient hypertensive patients. Vitamin D deficient are more common in overweight and female hypertensive patients, which may increase risk of atherosclerosis, so monitoring of hs-CRP has predictor values.
Keywords: hs-CRP, Hypertension, Cardiovascular disease, Vitamin D, Sudan
Cite this paper: Rawia Abdalla Mohamed , Muzamil Mohammed Musa , Amar Mohamed Ismail , High Sensitive C-Reactive Protein(hs-CRP) as Predictor Marker for Cardiovascular Disease among Vitamin D Deficient Hypertensive Patients, American Journal of Medicine and Medical Sciences, Vol. 5 No. 1, 2015, pp. 1-6. doi: 10.5923/j.ajmms.20150501.01.
Article Outline
1. Introduction
- Hypertension (HTN) is defined as persistent systolic blood pressure (BP) of at least 140 mm Hg and/or diastolic pressure of at least 90 mm Hg, or BP that is controlled to guideline-recommended levels using antihypertensive medication [1-3]. High blood pressure (BP) is a major health problem throughout the world because of its high prevalence and its association with increased risk of cardiovascular disease [4]. In 2005, approximately 75 million people had high BP: 34 million males and 39 million females. Earlier studies of hypertension prevalence in the Sudan were estimated at 7.5% [5]. Essential hypertension is systemic hypertension of unknown cause that results from dysregulation of normal homeostatic control mechanisms of blood pressure in the absence of detectable known secondary causes over 95% of all cases of hypertension are in this category [2].For most primary care providers, hyperlipidemia is defined as elevations of fasting total cholesterol concentration which may or may not be associated with elevated TG Concentration [6]. Although Hyperlipidemia is present in only half of all patients who develop coronary heart disease [7], Health care providers are concerned about hyperlipidemia because of the well-established association between lipid concentrations and the risk of CVD, the leading cause of death in the United States [6]. Over the past several years evidence has accumulated that inflammatory mechanisms play a pivotal role in the genesis of atherosclerosis and thus its complications [7]. Atherosclerosis is an inflammatory disease. Because high plasma concentrations of cholesterol, in particular those of low-density lipoprotein (LDL) cholesterol, are one of the principal risk factors for atherosclerosis, the process of atherogenesis has been considered by many to consist largely of the accumulation of lipids within the artery wall [8]. Attention has thus focused on whether circulating markers of inflammation can provide a new method to improve cardiovascular risk prediction, one of these inflammatory markers is hs-CRP which has been the best studied. Produced in the liver in response to interleukin-6, hs-CRP has been shown to predict myocardial infarction, stroke and vascular death in a variety of clinical settings. hs-CRPalso appear to have predictive value in both stable and unstable angina as well as in the chronic phase after myocardial infarction, an intriguing finding in light of the emerging role of inflammation in determining plaque stability [7]. Resent research suggest that patients with elevated basal levels of CRP are at increased risk of diabetes, hypertension and cardiovascular disease [9]. It has been hypothesized that a high CRP levels might reflect a large benefit from statins. This is based on the JUPITER trial that found elevated CRP levels without hyperlipidemia benefited. Statins were selected because they have been proven to reduce levels of CRP [10]. A subsequent trial however failed to find that CRP was useful for determining statin benefit [11]. To clarify whether CRP is a bystander or active participant in atherogenesis, a 2008 study compared people with various genetic CRP variants. Those with a high CRP due to genetic variation had no increased risk of cardiovascular disease compare to those with a normal or low CRP [12].Vitamin D is a fat soluble vitamin with well-known functions in bone homeostasis and metabolism. Research carried out over recent decades indicates that vitamin D deficiency plays an important role in many non-skeletal diseases such as hypertension and CVD. The vitamin D receptor (VDR) is found within various tissues and the α1-hydroxy enzyme (which converts 25-hydroxyvitamin D (25(OH) D) to 1,25 (OH) 2-D, the active form of vitamin D) is found locally. The effect of vitamin D on regulation of the lipid profile is one of the proposed mechanisms for the relationship between vitamin D deficiency and CVD [13]. Since both vitamin D deficiency and inflammation, have been linked to cardiovascular diseases by the modulatory effect of vitamin D on rennin-angiotensin system (RAS) as well as its inhibitory effect on vascular smooth muscle hypertrophy [14]. Angiotensin II can contribute to atherogenesis by stimulating the growth of smooth muscle by binds to specific receptors on smooth muscle, resulting in the activation of phospholipase C, which can lead to increases in intracellular calcium concentrations and in smooth-muscle contraction, increased protein synthesis, and smooth-muscle hypertrophy. It also increases smooth-muscle lipoxygenase activity, which can increase inflammation and the oxidation of LDL [15]. Hypertension also has proinflammatory actions, increasing the formation of hydrogen peroxide and free radicals such as superoxide anion and hydroxyl radicals which reduce the formation of nitric oxide by the endothelium thus increase leukocyte adhesion [15]. Vitamin D may inhibit the Ras by reducing renin gene expression, increasing 1,25(OH)2D concentrations were associated with lower plasma renin activity in hypertension, both 25(OH)D and 1,25(OH)D were inversely associated with plasma renin and angiotensin II concentrations [15, 16].
2. Materials and Methods
- In this cross-sectional study, 176 diagnosed as hypertensive patients aged 21 to 80 years (92 males and 84 females) from different Sudanese hospitals were included. Hypertensive patients with inflammation, autoimmune diseases, diabetes, renal diseases and/or hypertensive under vitamin D supplementation were excluded from this study. Overnight fasting (5 ml) peripheral blood was withdrawn. Serum was obtained by centrifugation of blood at 3000 rpm for 10 min and stored at −20°C.
2.1. Ethical Consideration
- The study has been approved by the local ethics committee of Al-Neelain University. All participants in the study were given their written informed consent considering the aims of the study and sample and clinical information’s were used anonymously.
2.2. Measurement of BMI
- Anthropometric data including weight and height were measured thus body mass index (BMI) was defined as weight (kg) divided by height squared (m2).
2.3. Estimation of Vitamin D
- Briefly according to manufactured, vitamin D Serum levels were measured using the competitive inhibition Enzyme linked immune sorbent assay or ELISA (EUROIMMUN, AG German). Sample (200 μl) was diluted with biotin in microplate well which coated with monoclonal anti vitamin D antibodies, during incubation an unknown amount of 25-OH vitamin D were competed for the antibody sites while unbound 25-OH vitamin D was removal by washing. Streptavidin-peroxidase (100 μl) was added to detect bound biotin labeled 25-OH vitamin D then peroxidase substrate tetramethylbenzidine (TMB) (100 μl) was added to promote a color reaction. The color intensity was inversely proportional to the 25-OH vitamin D concentration in the sample. Samples results were calculated by using standard curve (SUNRISE-TECAN).
2.4. Estimation of hs-CRP
- According to the procedure provided, serum levels of Hs-CRP were measured using the Particle enhanced immunoturbidimetric assay method (Cobas C 311 automated chemistry analyzer). Human CRP agglutinates with latex particles coated with monoclonal anti-CRP antibodies. The precipitate is determined turbidimetrically, samples dispensed and all processes done automatically and concentration obtained for each sample.
2.5. Estimation of Lipid Profile
- Triglycerides was catalysed by glycerokinase peroxidise peroxidase method through sequences of enzymatic catalysis steps by lipases, triglyceride catalyzed yield H2O2 which oxidized to yield colour dye of quinonimine, the absorbance increase directly proportional to the concentration of triglycerides. Cholesterol also catalyzed to yield H2O2 which oxidized for amino anti-pyrine with phenol to form colour dye of quinoneimine, the absorbance increase directly proportional to concentration of cholesterol were measured spectrophotometrically using BTS-310 Biosystem. Briefly LDL-C and HDL-C were measured using precipitation methods by the same principle and procedure used for cholesterol estimation.
2.6. Statistical Analysis
- The Student’s t-test was employed to compare differences between the means of continuous variables. P-values less than 0.05 were considered statistically significant. Data were analyzed by SPSS statistical package of social science (version 16.0; SPSS Inc.).
3. Results
3.1. Study Variables in Case and Control Groups
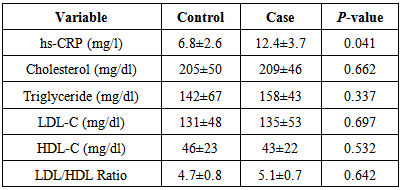
- The results showed mean hs-CRP level was significantly higher in case (12.4±3.7 mg/l) compared with control (6.8±2.6 mg/l) with p-value 0.041, in addition all mean lipid parameters level showed insignificant differences when compared case with control groups (Table 1).
3.2. Study Variables among Gender
- In our study 176 hypertensive patients 92 (52.3%) were males 82 (47.7%) were females. The percentage of overweight is slightly higher in male 80.4% than in female 66.7%., in contrast hypertensive male tend to have normal vitamin D level (30 ± 3.3 ng/ml) in comparison with female which tend have vitamin D deficient (14.6 ± 1.6 ng/ml) with (p-value 0.000). In addition lipid profile showed insignificant differences among gender (Table. 2).
|
3.3. Study Parameters in Groups Classified according to BMI
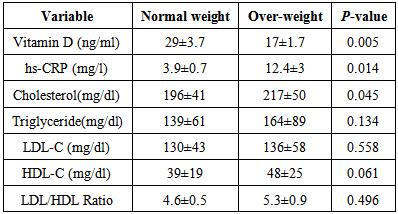
- Results showed overweight subjects (17±1.7 ng/ml) tend to have vitamin Ddeficient more than normal weight (29±3.7 ng/ml) with p-value 0.005, whereas hs-CRP was significantly higher in overweight (12.4±3 mg/l) than normal weight (3.9±0.7 mg/l) with p-value 0.014, also cholesterol was significantly increased in overweight (217±50 mg/dl) in comparison with normal weight (196±41 mg/dl) with p-value 0.045. All others parameters showed insignificant difference (Table 3).
|
3.4. Study Parameters among Groups Classified according to Duration of the Hypertension
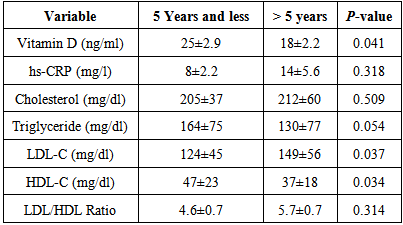
- The results showed mean vitamin D and HDL-C level were significantly lower in >5 years duration of hypertension compared with those <5 years duration of hypertension with p-value 0.014 and 0.034 respectively. LDL-C was significantly higher in>5 years of hypertensive compared <5 years duration of hypertension with p-value 0.037. Whereas all others parameters showed insignificant difference (Table 4).
|
4. Discussion
- Previous studies reported that hypertension is in part an inflammatory, therefor observed an elevated levels of CRP in hypertensive individuals, which hypothesize that CRP may induce a decrease in endothelium dependent relaxation thus a potential risk factor for hypertension, reverse causation might also be implicated, whereby high blood pressure may induce inflammation and raise CRP levels [17-20]. Since both vitamin D deficiency and inflammation have been linked to cardiovascular disease, by the modulatory effect of vitamin D on rennin-angiotensin system as well as its inhibitory effect on vascular smooth muscle hypertrophy [14]. Accordingly the results of present study showed significant increase in hs-CRP of case in comparison with control group with (p-value 0.041). In fact that hs-CRP is marker for cardiovascular disease our results indicates hs-CRP could by useful predictor marker for cardiovascular disease in deficient hypertensive patients. Hence insufficient vitamin D status may lead to impaired immune function, with an increased vulnerability to proinflammatory state, by mechanism of decrease concentrations of inflammatory cytokines like TNFα and IL-6, while increasing secretion of the anti-inflammatory cytokine IL-10. In addition hypertensive elderly patients showed significant negative associations between vitamin D and the pro-inflammatory markers IL-6 and CRP, similar observation was made that, vitamin D deficiency is independently associated with elevated hs-CRP, but this observation was made on children and young adults with lupus erythematosus [17, 21, 22].The results of independent t-test showed mean vitamin D level was significantly lower in female than male with (P-value 0.000), thus females were more susceptible to vitamin D deficiency than males, the possible justification for our results is that males spend more time outdoors and women wear sun protective clothing and avoid sun exposure which may affect vitamin D synthesis [23-26]. In contrast the results of hs-CRP showed insignificant difference concerning to gender with (P-value 0.374). This finding was agreed with studies performed in Nigeria and India [17, 19]. In this study the results of mean concentration of cholesterol, triglyceride, LDL, HDL and LDL/HDL Ratio revealed insignificant differences when compared male with female with P-values (0.257, 0.390, 0.128, 0.890 and 0.273) respectively in gender variation.To our knowledge the current research is the first study link between hypertension, vitamin D, CRP and BMI, which reported there was significant decrease in mean vitamin D level of overweight in comparison with normal weight groups with (P-value 0.005). in fact previous studies reported obese subjects had significantly lower basal 25-hydroxyvitamin D concentrations than did age-matched control subjects, the prevalence of vitamin D deficiency was highest in individuals with BMI ≥40, being as high as 32% among women and 46% among men, this indicates that obesity-associated vitamin D insufficiency is likely due to the decreased bioavailability of vitamin D from coetaneous and dietary sources because of its deposition in body fat compartments, this apparent decrease in vitamin D bioavailability with increased adiposity has been hypothesized to be due to the increased sequestration of vitamin D in fat, because vitamin D is fat soluble and is readily stored in adipose tissue, it could be sequestered in the larger body pool of fat of obese individuals [27-30]. In addition significant increase in mean hs CRP level in overweight compared with normal weight group with (P-valve 0.014). The production of CRP is regulated by cytokines, principally interleukin-6 (IL-6), and serum CRP levels reflect IL-6 activity in humans. It was demonstrated that IL-6 is released in vivo by subcutaneous adipose tissue and is thereby able to have systemic effects, particularly in obese subjects, thus, adipose tissue may play a role in the regulation of serum CRP concentrations via IL-6 production [31]. Our results were confirmed by previous studies found that hs-CRP level is high in obese patients and there was close relationship between BMI and hs-CRP serum levels, thus serum CRP concentrations were significantly correlated with BMI [31, 32]. In addition the results revealed there were significant differences between cholesterol concerning BMI (p-Value (0.045), whereas no significant differences in others lipid parameters triglyceride, LDL, HDL and LDL/HDL Ratio, P-values (0.134, 0.558, 0.061 and 0.496) respectively.Also our study showed there was significant increase in the mean of vitamin D level in >5 years duration compared with <5 years duration of disease with (P-value 0.041). The majority of observational data suggest that lower levels of vitamin D may be associated with a higher blood pressure and a higher risk of developing hypertension [16]. Among interventional studies, there are a studies supporting the idea that vitamin D supplementation reduces blood pressure. In contrast, there are studies claiming that vitamin D is not correlated with blood pressure. It seems that difference in dose and duration of vitamin D supplementation are the reason for this contradiction [14].
5. Conclusions
- In conclusion hs-CRP was higher in vitamin D deficient hypertensive patients and since hs-CRP is a marker for atherosclerosis thus it could be useful predictor marker for cardiovascular disease in vitamin D deficient hypertensive patients. Vitamin D deficient is more common in hypertensive females thus are more susceptible to atherosclerosis and cardiovascular diseases, thus hs-CRP and lipid profile should be monitored regularly.
References
| [1] | Sobh M.A, 2000, Hypertension and the Kidney, Essentials of Clinical Nephrology, 1st Edition, 285-296. |
| [2] | Rosendorf C, 2005, Hypertension: Mechanisms and Diagnosis, Essential Cardiology Principles and Practice, 2nd Edition, 595-620. |
| [3] | Bishop M. L, Fody E. P, Schoeff L. E, 2010, Clinical Chemistry Techniques, Principles, Correlations, 6th Edition. |
| [4] | El-Guindy M. S, 2005. Definition and classification, Clinical guidelines for the management of hypertension, series 29, 11-55. |
| [5] | Elzubier A. G, Husain A. A, Suliman I.A, 2000. Drug compliance among hypertensive patients in Kassala, Eastern Sudan, Eastern Mediterranean Health Journal, Volume 6:100-105. |
| [6] | Nelson RH, 2013, Hyperlipidemia as a Risk Factor for Cardiovascular Disease, Prim Care, Volume 40: 195–211. |
| [7] | Blake G. J. and Ridker P. M, 2001, High sensitivity C-reactive protein for predicting cardiovascular disease: an inflammatory hypothesis, European Heart Journal, Volume 22, 349–352. |
| [8] | Ross R, 1999, Atherosclerosis—an inflammatory disease, N Engl J Med, Volume 340: 115–126. |
| [9] | Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM, 2001, C-reactive protein, Interlukin-6, and Risk Developing Type 2 Diabetes mellitus, Journal of American Medical Association( JAMA), Volume 286:327-334. |
| [10] | Ridker PM, Danielson E, Fonseca FA,Genest J, Gotto AM, Kastelein JJ, Koening W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, 2008, Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein, The New England Journal of Medicine, Volume 359: 2195-2207 . |
| [11] | Emberson J, Bennett D, Link E, Parish S, Danesh J, Armitage J, Collins R, 2011, C- reactive protein concentration and the vascular benefits of statin therapy: an analysis of 20,536 patients in the Heart Protection Study, Lancet, Volume 377: 469-476. |
| [12] | Zacho J, Tybjærg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG, 2008, Genetically Elevated C-Reactive Protein and Ischemic Vascular Disease, The New England Journal of Medicine, Volume 359:1897-1908. |
| [13] | Saedisomeolia A., Taheri E., Djalali M., Malek A., Moghadam S., and Qorbani M, 2014, Association between serum level of vitamin D and lipid profiles in type 2 diabetic patients in Iran, Journal of Diabetes & Metabolic Disorders, Volume 13:7. |
| [14] | Jafari T, Paknahad Z, 2014, Vitamin D and Hypertension, Zahedan Journal of Research in Medical Sciences, Volume 16: 1-7. |
| [15] | Wang L, 2009, Vitamin D and Hypertension, North American Journal of Medicine & Science, Volume 2: 149-151. |
| [16] | Vaidya A, Forman J.P, 2010, Vitamin D and Hypertension, Hypertension Journal, Volume 56: 774-779. |
| [17] | Dar MS, Pandith AA, Sameer AS, Sultan M, Yousuf A, Mudassar S, 2010, hs-CRP: A Potential maker for hypertension in Kashmiri population, Indian Journal of Clinical Biochemistry, Volume 25: 208-212. |
| [18] | Ingle PV, Patel DM, 2011, C- reactive protein in various disease condition – An overview, Asian Journal of Pharmaceutical and Clinical Research, Volume 4: 9-13. |
| [19] | Idemudia JO, Idogun ES, 2012, High sensitive C-reactive protein (HsCRP) as a cardiovascular risk factor in hypertensive Nigerians, The Nigerian Postgraduate Medical Journal, Volume 19: 163-166. |
| [20] | Yanchun D, Wang Jian W, Zhang Pengqiang Z, Qu Peng Q, 2012, The relation of serum high-sensitive C-reactive protein to risk factors and target organ damage in hypertensive patients, Heart, Volume 98: 258-259. |
| [21] | Robinson AB, Tangpricha V, Yow E, Gurion R, McComsey GA, Schanberg LE, 2014, Vitamin D deficiency is common and associated with increased C-reactive protein in children and young adults with lupus: an Atherosclerosis Prevention in Pediatric Lupus Erythematosus substudy, Lupus Science & Medicine, Volume 1: 1-7. |
| [22] | Laird E, McNulty H, Ward M, Hoey L, McSorley E, Wallace JM, Carson E, Molloy AM, Healy M, Casey MC, Cunningham C, Strain JJ, 2014, Vitamin D Deficiency Is Associated With Inflammation in Older Irish Adults, The Journal of Clinical endocrinology and metabolism, Volume 99: 2013-3507. |
| [23] | Nurbazlin M, Chee WS, Rokiah P, Tan AT, Chew YY, Nusaibah AR, Chan SP, 2013, Effects of sun exposure on 25(OH) vitamin D concentration in urban and rural women in Malaysia, Asia Pacific Journal of Clinical Nutrition, volume 22: 391-399. |
| [24] | Batieha A, Khader Y, Jaddou H, Hyassat D, Batieha Z, Khateeb M, Belbisi A, Ajlouni K, 2011, Vitamin D status in Jordan: dress style and gender discrepancies, Annals of Nutrition and Metabolism, Volume 58: 10-17. |
| [25] | Golbahar J, Al-Saffar N, Diab DA, Al-Othman S, Darwish A, 2013, Vitamin D Status in Adults: A Cross Sectional Study, Bahrain Medical Bulletin, Volume 35: 1-11. |
| [26] | Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, Hollis BW, Drezner MK, 2007, Low Vitamin D Status despite Abundant Sun Exposure, The Journal of Clinical endocrinology and metabolism, Volume 92:2130-2135. |
| [27] | Worestman J, Matsuoka L.Y, Chen TC, Lu Z, Holick MF, 2000, Decreased bioavailability of vitamin D in obesity, Journal of American Society for Clinical Nutrition, Volume 72:690-693. |
| [28] | Parikh S.J, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA , 2004, The Relationship between Obesity and Serum 1,25-Dihydroxy Vitamin D Concentrations in Healthy Adults, Journal of Clinical endocrinology and metabolism, Volume 89:1196-1199. |
| [29] | Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J, 2009, The Dependency of Vitamin D Status on Body Mass Index, Gender, Age and Season, Anticancer Research, Volume 29: 3713-3720. |
| [30] | Tsiaras WG, Weinstock MA, 2011, Factors Influencing Vitamin D Status, Acta Dermato-Venereologica, Volume 91: 115-124. |
| [31] | Bastard JP, Jardel C, Delattre J, Hanique B, Bruckert E, Oberlin F, 1999, Evidence for a Link Between Adipose Tissue Interleukin-6 Content and Serum C-Reactive Protein Concentrations in Obese Subjects, Circulation, Volume 99: 2219-2222. |
| [32] | Gokalp, Tuzcu A, Akay H, Arikan S, Bahceci M, 2007, The association of high sensitivity C-reactive protein levels with body fat mass and body fat distribution, Endocrine Abstracts, Volume 14: 238. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML