Azza M. Youssef, Atef F. Shata, Hesham M. Kamal, Yasser El-Saied, Omaima F. Ali
MD of Obstetrics and Gynecology, Consultants of Obstetrics and Gynecology, El Mataria Teaching Hospital
Correspondence to: Azza M. Youssef, MD of Obstetrics and Gynecology, Consultants of Obstetrics and Gynecology, El Mataria Teaching Hospital.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Iron deficiency anemia in women of reproductive age group is a major health problem in Egypt. Objective:The present study was designed to compare the efficacy and tolerability of three oral iron preparations to treat anemic pregnant women. Methodology: The pregnant women (n=300) were divided into 3 groups of pregnant women (n=100). After 13 weeks of gestation, each group was treated with one type of oral iron. first group, fully reacted amino acid chelation minerals ferrous bisglycinate (27 mg), second group, iron multi amino acid chelate (30 mg 2 tab/day), and third group, ferrous fumarate (198mg 3 tab/day) respectively. Follow-up was done for one month. Hemoglobin level (Hb), mean corpuscular volume (MCV), mean corpuscular heamoglobin concentration (MCHC), reticulocyte count (RC), and serum ferritin were assessed after 4 weeks. Adverse effects were monitored at end of one month. Results: A significant increase in Hb was seen in all three groups (p<0.05). Increase in ferritin with ferrous bisglycinate (27 mg) was significantly more than other treatments (p<0.05). Nausea, vomiting, epigastric pain did not significantly coexist with ferrous bisglycinate (27mg). Conclusion: It can be concluded that ferrous bisglycinate (27mg) can be considered the best effective medication with tolerable side effects for treatment as well as prevention of iron deficiency anemia in pregnancy.
Keywords:
Iron Deficiency Anemia, Safety, Pregnant Women, Ferrous Bisglycinate, Iron Multi Amino Acid Chelate, Ferrous Fumarate
Cite this paper: Azza M. Youssef, Atef F. Shata, Hesham M. Kamal, Yasser El-Saied, Omaima F. Ali, A Comparative Study of Efficacy, Tolerability, and Compliance of Oral Iron Preparations for Iron Deficiency Anemia in Pregnant Women, American Journal of Medicine and Medical Sciences, Vol. 4 No. 6, 2014, pp. 244-249. doi: 10.5923/j.ajmms.20140406.09.
1. Introduction
Iron deficiency continues to be one of the most prevalent single-nutrient deficiencies in the world. Intervention is often designed to inhibit the decrease of hemoglobin concentration and the decline in iron stores associated with pregnancy. Enrichment and fortification of food items proved with some success in developed countries, but not often in the developing world. [1]Iron deficiency anemia in pregnancy is the commonest type of anemia; it causes both maternal and fetal complications. Women with a hemoglobin level below 10 g/dl are considered to have an accepted anemia. Serious maternal consequences directly related to the anemia are rare in women with levels between 6 and 10 g/dl although the process may lead to serious morbidity. Indirectly, it may be related to obstetric complications such as postpartum hemorrhage, operative delivery, and placental abnormalities (abruptio placentae or placenta previa). [2] Morbidity, such as infections, prolonged hospital stays, and general recuperative health problems, has also been associated with preexistent anemia. On the other hand, patients with hemoglobin levels below 4 to 6 g/dl may face life threating problems owing to high output congestive heart failure with decreased oxygenation of cardiac tissue.Anemia has been associated with prematurity, low-birth-weight infants, abortions and fetal deaths, even when the process is mild (hemoglobin levels 8 to 11 g/dl). Moreover, mild deficiency states have been related to decreased birth weight or fetal loss. [2].Oral iron preparations for the correction of iron deficiency include iron salts, Iron multi amino acid chelate and Ferrous bisglycinate. Iron salts like ferrous fumarate is extensively prescribed for the prevention and treatment of iron deficiency. Results from iron supplementation studies have shown that iron absorption from iron amino acid chelate preparations are superior to that of iron salts preparations [1].Moreover, the bioavailability of iron preparation increases with the increasing dose of ferrous fumarate [3]. However, gastrointestinal symptoms such as nausea, epigastric pain and constipation are commonly associated with iron salts. Food and / or chelating drugs in the gastrointestinal tract may interfere with absorption and decrease the concentration of the bioavailable iron [4, 5]. This leads to variability in the Hb correction during anemia in pregnancy.Now a different new fully reacted amino acid chelation, ferrous bisglycinate 27 mg (Chelation is a process where amino acids are attached to a mineral); in this case ferrous bisglycinate is attached to two molecules of the amino acid glycine by a covalent bound. [6]Ferrous bisglycinate 27 mg is marketed and claimed to have low gastrointestinal intolerance and therefore better patient compliance. Few of these newer preparations are also claimed to increase Hb level faster as well as improve iron storage better than conventionally used ferrous fumarate. Therefore, it was thought worthwhile to study the effects of one standard conventional oral iron preparation – ferrous bisglycinate– as an amino acid chelate that has shown great efficacy, less GI irritation, and more absorption that is not retarded by the presence of phytates. [9]
2. Material and Methods
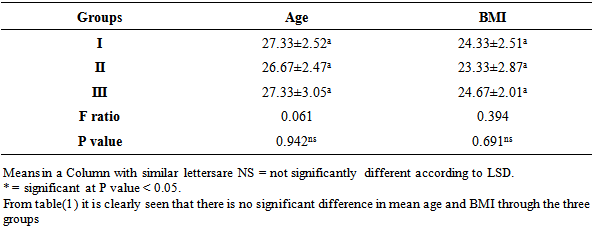
Study sample: The study was conducted in EL-Mataria Teaching Hospital, Cairo, Egypt over 1year from april 2013 till march 2014. It was designed as a prospective longitudinal study with 300 pregnant women who were attending out –patient clinic, their ages ranged 18–40 years, that participated in a prenatal check up program after 13 weeks gestation having Hb less than 9 gm/dl and that were diagnosed microscopically to have microcytic hypochromic anemia. The study protocol was approved by the institutional ethics committee at El-Mataria Teaching Hospital. After informing, written authorization was taken from the patients, they were randomly allocated into 3 groups (each n=100). The duration of study for each patient was 30 days (one month). The dropout patients were replaced by new patients in the study.Exclusion criteria: Pregnant women with Hb<7gm/dl, history of severe oral intolerance, excessive emesis, bleeding piles , active pepticulcer, high obstetric risk associated with hypertension, diabetes, hepatic, renal diseases or any other GIT problemsMedication: Study medication was supplied in the form of tablets.Group I: received a combination of ferrous bisglycinate containing 27mg elemental iron once daily.Group II: received a combination of iron multi amino acid chelate containing 15mg elemental iron twice daily.Group III: received a combination of ferrous fumarate containing 66mg elemental iron three times daily.After recruitment, the patients were supplied with the respective medication and asked to follow-up after week (7 days). During each follow-up visit, they were subjected to general and obstetric examination and supplied with study medication for the next 3 weeks. Compliance was checked by verbal enquiry and verified by checking empty or used packets of the drug brought in by the patients. Patients were also informed and given a reminder on the phone of the date of the next visit as well as a confirmation of the need to adhere to the given medication.Investigations: Samples for blood investigation were collected at day 0 (before starting medication) and day 30 (end of month). The amount of blood collected at each visit was 4 to 5 ml. The parameters of Hb, MCV, MCHC, reticulocyte count, and Serum ferritin were assessed at day 0 and at the end of month. Parameters like Hb, MCV and MCHC were done on automatic cellcounter (sysmax). Reticulocyte count was done by slide method and serum ferritin was done by a biochemistry analyzer.Safety measures: Patients were trained to record and observe the adverse effects and instructed to report immediately if serious adverse reaction occurs. Any adverse events like metallic taste, epigastric distress, abdominal pain, nausea, vomiting, diarrhea and constipation, were recorded on a case record form. Also, during follow-up visits, patients were evaluated for the following symptoms associated with iron deficiency anemia, fatigue, malaise, loss of appetite, breathlessness, palpitation, giddiness, and irritability.Statistical methods: All statistical calculations were done using computer programs. Microsoft Excel version 10 and SPSS (statistical package for the social science version 20.00) statistical programs were used at 0.05, 0.01 and 0.001 levels of probability. [10] A comparison of the percentage was done using the chi square (X2) test, t-test, paired t- test, one way analysis of variance (ANOVA), and Post hoc-LSD tests. Results (the least significant difference) were presented using percentage, mean, ± standard deviation. The discriminant analysis was estimated to show the relationship of the physiological parameter to each other. [11]Table 1. Results of ANOVA for Comparison between groups as regards the mean values of age, and BMI and their statistical significance
 |
| |
|
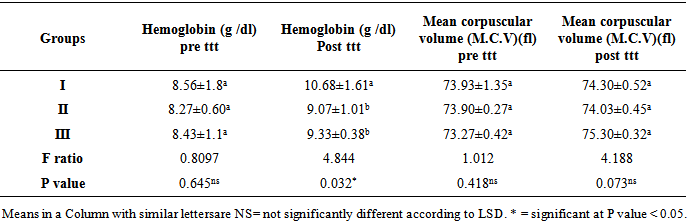
From table (2) it is clearly seen that there is no significant difference in mean Hb (g /dl) pre ttt, MCV pre ttt, between three groups (p>0.05). It is seen that in group II and group III, there is no significant increase in mean Hb(g /dl) post ttt at the end of month over the basal values (p >0.05). However in group I, there is only borderline increase in mean Hb (g /dl) post ttt, and MCV post ttt when compared to the two groups (II, III) (p< 0.05).Table 2. Results of ANOVA for Comparison between groupswith regards to the mean values of Hb (g /dl) (pre ttt,post ttt), (MCV)(pre ttt, post ttt) and their statistical significance
 |
| |
|
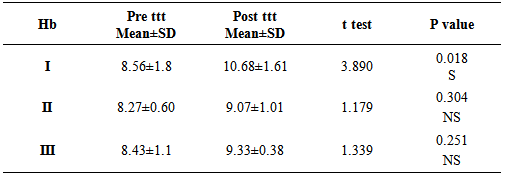
From table (3) it is clearly seen that there was significant increase in Hb in group I (ferrous bisglycinate) (p=0.018) versus non-significant increase in Hb in groups (II, III) (p = 0.304, 0.251).Table 3. Comparison between groups with regards to the mean values of Hemaglobin (pre ttt, post ttt)
 |
| |
|
From figure (1), Increase of 2.12 g/dL, 0.8 g/dL and 0.9 g/dL, in groups (I, II, III) respectively. Improvement in hemoglobin level for the ferrous bisglycinate product compared with ironmulti amino acid chelate product and ferrous fumarate product was statistically significant (P = 0.018).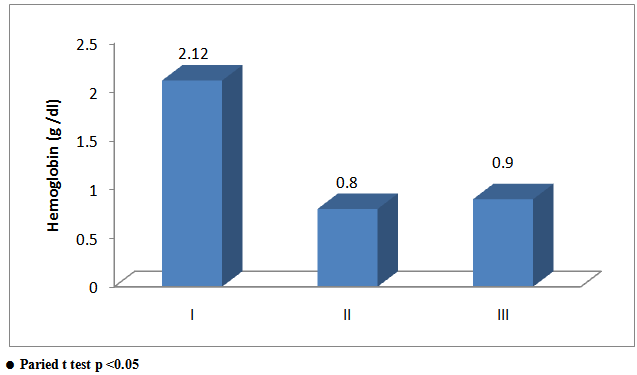 | Figure 1. Comparison between groups as regards Hb difference and their statistical significance |
From table(4) it is clearly seen that there is no significant difference in the mean reticulocyte count (%) Pre ttt, serum ferritin level (μg/ l) Pre ttt, MCHCPre ttt values over the basal values in all the three groups (p>0.05). It is seen that in group II and group III there is no significant increase in the mean reticulocyte count (%) post ttt , serum ferritin level (μg/ l) post ttt, MCHC post ttt at the end of month over the basal value (p >0.05). However in group I, there is only a borderline increase in the mean reticulocyte count (%) post ttt, serum ferritin level (μg/ l) post ttt, MCHC post ttt when compared to the two groups (II, III) (p< 0.05).Table 4. Results of (ANOVA) for comparison between groups with regards to the mean values of reticulocyte Count (%) (pre ttt, post ttt), serum ferritin level (μg/ l )(Pre ttt, post ttt), MCHC (Pre ttt, post ttt) and their statistical significance
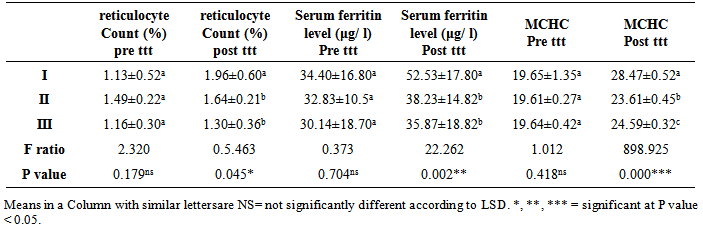 |
| |
|
There was significant increase in serum ferritin level in group I (ferrous bisglycinate) (p=0.044) versus non-significant increase in serum ferritin level in groups (II, III) (p = 0.666, 0.584) respectively. Thus, appreciable increases in iron stores are seen with group I (ferrous bisglycinate) (fig 2).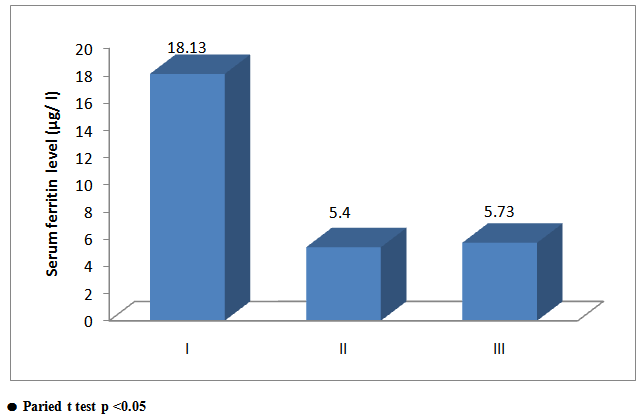 | Figure 2. Comparison between groups as regards serum ferritine and their statistical |
Table 5. Comparison between groups with regards to the mean values of serum ferritin level (μg/ l) (pre ttt, post ttt)
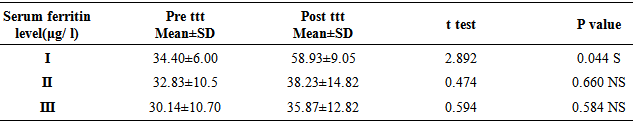 |
| |
|
Table 6. Side effects and discontinuation rates for the various iron-supplement Preparations
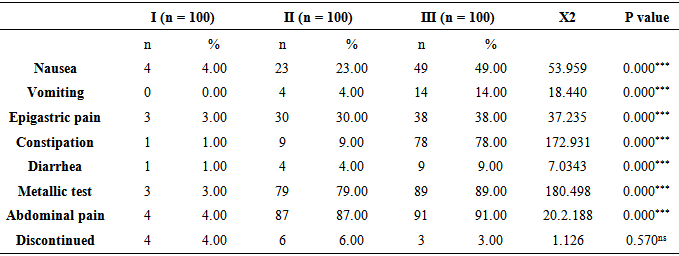 |
| |
|
The patient compliance was: Group I (fully reacted amino acid ferrous bisglycinate: 96%, Group II (iron multi amino acid chelate): 94%, Group III (ferrous fumarate): 97%. Adverse effect related to the iron preparation, This table shows a highly statistically significant difference (p <0.05) between the groups with nausea, vomiting, epigastric pain, constipation, diarrhea, metallic tests and abdominal pain. That indicates that group I has significantly lower side effects when compared with the other two groups, whereas when comparing between two groups (II, III) there are no statistically significant differences (p > 0.05) and cause ahighly significant side effect to the pregnant women.
3. Discussion
Iron deficiency anemia is very common worldwide. In Egypt, prevalence of iron deficiency anemia during pregnancy is high. Pregnancy with anemia has a significant impact on the health of fetus as well as that of the mother. [3] The treatment of iron deficiency anemia is given to replenish Hb and restore iron stores by supplying sufficient iron. In the present study, from a randomized single blind controlled trial involving pregnant anemic women, it was found that ferrous bisglycinate has efficacy in increasing Hb, MCV, MCHC, reticulocyte count, and serum ferritin. Overall adverse effects with ferrous bisglycinate, were low. It also has good compliance and low discontinuation rate.Another study Ferrous bisglycinate chelate it is more stable and has high bioavailability to ensure less GIT irritation and so that absorption of bisglycinate is not affected by phytates in food. [6] ferrous bisglycinate chelate has been used successfully for iron fortification. As a supplement it has been tested in many countries and has shown a great efficacy in reducing iron deficiency anemia. [13]On our study iron multi amino acid chelate and ferrous fumarate show no significant increase in the mean Hb, reticulocyte count (%), serum ferritin level (μg/ l), MCHC at the end of month and have high adverse effects when compared to ferrous bisglycinate.Many studies have shown that treatment with ferrous bisglycinate resulted in significant increase in hemoglobin (Hb) levels, the mean increase in Hb of the group receiving ferrous bisglycinate (22.72%) was significantly higher than that of the group receiving ferrous sulphate (18.66%). [2, 12, 15]Another study which involved pregnant women with iron deficiency anemia and which demonstrated comparable efficacy of ferrous bisglycinate, iron chelated, and Fe salt found ferrous bisglycinate to be more effective than Fe salt with less adverse effects. [14]Supplementation with conventional iron salt show low efficacy for the control of iron deficiency anemia due to poor compliance with the treatment because of its disagreeable flavor and adverse effects such as nausea, vomiting, constipation, diarrhea, and abdominal pain. [5]In a comparative study between ferrous bisglycinate and ferrous sulphate, it is reported that both group showed significant increase in Hb level, and the group which was administered ferrous bisglycinate had a significant rise in plasma ferritin. [13] Due to the additional demand for iron during pregnant women of child bearing age face a higher risk of iron deficiency. A preponderance of women (89%) who are prescribed preparations of iron salts, due to iron deficiency, eventually discontinue their regimen. The primary reasons for noncompliance related to uncomfortable side effects. The lowest (9.1%) discontinuation rates have been previously reported for the supplements containing iron chelates [16]. Ideally a supplementation with a well-accepted iron compounds accompanied with proper patient education could improve the compliance of iron supplementation resulting in a significant reduction in the prevalence of iron deficiency anemia. Among the possible alternatives to control iron deficiency
4. Conclusions
Ferrous bisglycinate has tolerable adverse effects, and it increases iron stores significantly. Ferrous bisglycinate also increases Hb and produces less adverse effects than the Iron multi amino acid chelate and ferrous fumarate. Thus, ferrous bisglycinate still can be considered effective treatment for prevention of iron deficiency anemia in pregnancy for treatment as well as prevention of iron deficiency anemia in pregnancy. Show fig (3).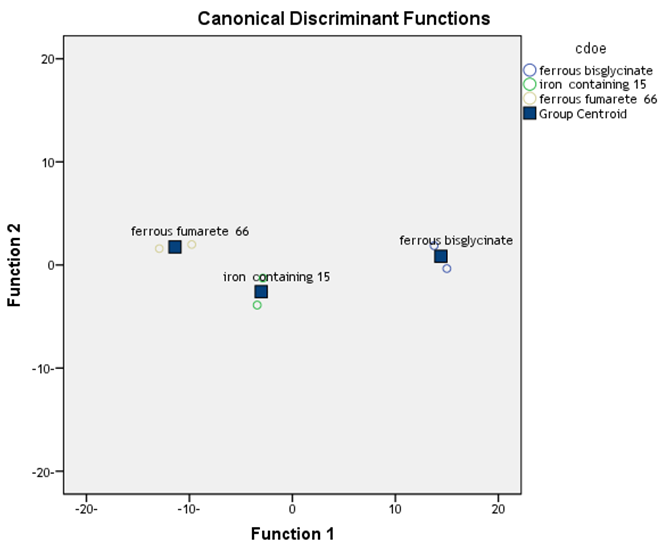 | Figure 3. Discremnant analysis show statistical difference btween groups |
5. Recommendations
All pregnant women after 13 weeks should be supplied with ferrous bisglycinate which is more efficient in increasing Hb, MCV, MCHC and iron stores. At the same time, it has tolerable adverse effects, high compliance, and low discontinuation rate.
References
| [1] | John L. Beard (2000): Effectiveness and strategies of iron supplementation during pregnancy, Am J. Nutr 71; 1288s-9. |
| [2] | Kitay D.Z. (1973): Assessing anemia in the pregnant patient. Contempob/Gyn 2;17. |
| [3] | Shatrugna V, Raman L and Kailash U (1999): Effect of doseand formulation on iron tolerance in pregnancy. NatlMed J India.12:18-20. |
| [4] | Geisser P and Muller A (1987): Pharmacokinetics of iron salts and ferric hydroxide-carbohydrate complexes. Arzneimittelforschung37:100-4. |
| [5] | Kaltwasser JP, Werner E and Niechzial M. (1987): Bioavailability and therapeutic efficacy of bivalent and trivalent iron preparations. Arzneimittelforschung. 37:122-9. |
| [6] | Pineda O, Ashmead HD, Perez JM. (1994): Effectiveness of iron amino acid chelate on the treatment of iron deficiency anaemia. J ApplNutr 1994; 46:2-11. |
| [7] | Saha L, Pandhi P, Gopalan S, Malhotra S, Shah PK. (2007): Comparison of Efficacy, Tolerability, and Cost of Iron Polymaltose Complex With Ferrous Sulphate in the Treatment of Iron Deficiency Anemia in Pregnant Women. Med Gen Med 9(1):1. |
| [8] | Hansen CM. (1994): Oral iron supplements. AM Phar S34: 66-71. |
| [9] | Snedecor, G. M. & Cochran, W. G. (1982): Statistical methods-7th edition,l owa state Univ., Press, Ames., loww3a, USA., pp. 325-330 |
| [10] | Härdle, W. & Simar, L. (2007): Applied Multivariate Statistical Analysis. 2nd ed, Springer, 420pp. |
| [11] | Singh S, Singh S, Subhadra Lata, Jain R, Bissa U, T. Ramanidevi, (2011): Evaluation of efficacy and tolerability of ferrous bisglycinate tablets in comparison with ferrous sulphate tablets in patients with iron deficiency anemia. Journal of Pharmacy Research 4(8): 2836-2839. |
| [12] | Jeppsen RB, Borzelleca JF. (1999): Safety evaluation of Ferrous bisglycinate. Food and chemical toxicology 37: 723-731. |
| [13] | Szarfarc SC, Luz Marina Nunez de cassana, Fujimori E. (2001): Relative effectiveness of iron bis-glycinate chelate and ferrous sulfate in the control of iron deficiency in pregnant women. Archivoslatinoamericanos De Nutricion 51(1):42-47. |
| [14] | Sharma JB and Nagabhushan KH. (2012): Efficacy of Ferrous bisglycinate (FeroseTM) in anemia during pregnancy. Journal of Pharmacy Research 5(5),2881-2882 |
| [15] | Melamed N; Ben-Haroush A; Kaplan B and Yogev (2007); Y. Iron supplementation in pregnancy–does the preparation matter? Arch Gynecol Obstet. 276(6):601–604. |
| [16] | Hertrampf E and Olivares M. (2004): Iron amino acid chelates. Int J VitamNutr Res. 74(6):435–443. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




