-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2014; 4(6): 223-229
doi:10.5923/j.ajmms.20140406.05
Influence of Previous Moderate Training on the Elevated Serum Levels of Growth Factors and Inflammatory Mediators in Rats Exposed to Acute Exhausting Physical Exercise
Yahya M. Naguib1, Rania M. Azmy2
1Clinical Physiology Department, Faculty of Medicine, Menoufia University, Menoufia, Egypt
2Medical Biochemistry Department, Faculty of Medicine, Menoufia University, Menoufia, Egypt
Correspondence to: Yahya M. Naguib, Clinical Physiology Department, Faculty of Medicine, Menoufia University, Menoufia, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Appropriate physical exercise improves both mental and physical health. However, strenuous exercise can cause muscle damage and inflammation. The mechanism of muscle adaptation to physical exercise is in some way unclear. The aim of the present study was to evaluate the influence of previous moderate training on the generation of growth factors and inflammatory cytokines following an acute bout of exhausting physical exercise. Forty eight male Wistar albino rats were used in the present study. Rats were randomly assigned (24/group) to untrained (UT), and trained (T, subjected to 6 week swimming training) groups. Each group was equally subdivided into pre- and post-exercise groups. Blood samples were collected either immediately before or after acute exhausting swimming exercise session (60 minutes continuous swimming). Strenuous exercise induced a significant increase (P < 0.05) in serum levels of transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), interleukin 6 (IL-6), interleukin 1 beta (IL-1β) and tumour necrosis factor alpha (TNF-α) in (UT) and (T) rats when compared to the corresponding baseline values. Exercise-induced increase in the circulating levels of growth factors and inflammatory cytokines was significantly (P < 0.05) lower in the (T) group when compared to the (UT) group. We concluded that prior moderate exercise training protects against the deleterious effects of acute strenuous physical exercise by influencing exercise-induced rise in the serum growth factors and inflammatory cytokines.
Keywords: Exercise, Muscle plasticity, TGF-β, VEGF, TNF-α, Interleukins
Cite this paper: Yahya M. Naguib, Rania M. Azmy, Influence of Previous Moderate Training on the Elevated Serum Levels of Growth Factors and Inflammatory Mediators in Rats Exposed to Acute Exhausting Physical Exercise, American Journal of Medicine and Medical Sciences, Vol. 4 No. 6, 2014, pp. 223-229. doi: 10.5923/j.ajmms.20140406.05.
Article Outline
1. Introduction
- Regular physical exercise such as walking, running, or swimming is widely accepted to have a beneficial impact in health and disease. Habitual physical activity prevents the development of coronary artery disease (CAD), improves the condition of patients with established cardiovascular disease (CVD), and reduces the risk of other chronic diseases such type II diabetes, osteoporosis, obesity, depression and cancer [1-5]. However, published data also suggest that physical exercise induces muscle damage and non-specific inflammatory response, which were manifested by elevated concentrations of circulating pro-inflammatory growth factors and cytokines [6-8]. Growth factors and cytokines are potent intercellular signalling molecules which act within the local tissues in an autocrine or paracrine manner. However, systemic spillover of pro-inflammatory growth factors and cytokines such as transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), interleukin 6 (IL-6), interleukin 1 (IL-1), tumour necrosis factor alpha (TNF-α) from damaged tissues has been detected. Elevated serum levels of growth factors and hypercytokinaemia have been reported in a variety of serious insults such as severe trauma, burns, hemorrhagic shock, sepsis and ischaemia-reperfusion injuries [9, 10].TGF-β belongs to a family of dimmers that comprises 3 isoforms: TGF-β1, TGF-β2 and TGF-β3. Although TGF-β is a growth inhibitor to most epithelial cells in the culture, yet its effect on fibroblasts and smooth muscle cells is concentration-dependent. TGF-β is indirectly mitogenic at lower concentrations, while at higher concentrations it acts as a growth inhibitor [6]. TGF-β is also known to regulate embryonic development, angiogenesis, collagen production and degradation. TGF-β is a pleiotropic multifunctional cytokine with prominent anti-inflammatory effects [11, 12]. It has been reported that TGF-β can block NK cell proliferation and cytotoxicity. TGF-β can also inhibit the induction of IL-12 and natural killer (NK) cell interferon gamma (IFN-γ) production [13]. Intriguingly, TGF-β also has evident pro-inflammatory effects which are overlapping with those of IL-8 [14]. Both TGF-β and IL-8 are chemotactic for granulocytes and they enhance their phagocytic and bactericidal functions [15]. VEGF belongs to the family of at least 6 isoforms. Hypoxia is the main stimulus for VEGF production and expression. TGF-β, platelet derived growth factor (PDGF), IL-6 and IL-1, are also potent stimulators for VEGF production. VEGF stimulates endothelial cells proliferation and differentiation, increases vascular permeability, prevents endothelial apoptosis, regulates vasodilatation, and promotes angiogenesis in cancer, chronic inflammatory states and in healing wounds [16].IL-6, IL-1 and TNF-α are classical pro-inflammatory cytokines. They provoke pyrogenesis, neutrophilia and lymphocytopenia. They promote these acute inflammatory responses by the induction of IL-8, IFN-γ and monocyte chemotactic protein 1 (MCP-1) [9, 17]. The circulating plasma concentrations of the classical pro-inflammatory cytokines IL-6, IL-1 and TNF-α increase after physical exercise depending on the intensity and duration of endurance exercise, and decline in the post-exercise period [10, 18, 19]. IL-6 is the first cytokine to be present in the circulation during exercise. Contracting skeletal muscle is the main source of IL-6 in the circulation. In response to exercise, IL-6 mRNA increases in the contracting skeletal muscle and may reach up to 100-fold increases at the end of the exercise bout. The increase in IL-6 mRNA content could be detected after 30 minutes of exercise [20-22]. It was also demonstrated that both muscle IL-6 mRNA expression and protein release are enhanced by low glycogen level, indicating that IL-6 is involved in energy metabolism during endurance exercise [19, 23, 24].Physical exercise influences muscle plasticity; adaptive changes in skeletal muscle composition and metabolism. The mechanism underlying this phenomenon is not completely clear yet. The possibility of the involvement of TGF-β, VEGF, IL-6, IL-1β and TNF-α in the regulation of muscle plasticity phenomenon arises from the fact that their activity increases after physical exercise. The aim of the present study was to investigate the influence of previous moderate exercise training on the exercise-induced rise in serum TGF-β, VEGF, IL-6, IL-1β and TNF-α level following an acute bout of exhausting physical exercise.
2. Materials and Methods
- AnimalsThe experimental procedures were conducted in adherence to the Guiding Principles in the Use and Care of Animals published by the National Institutes of Health (NIH Publication No 85–23, Revised 1996). Animal care and use was approved by the University Ethics Committee. Animals were kept for 10 days prior to the start of the study to allow proper acclimatization. The animals were fed standard laboratory chow and allowed free access to water in an air-conditioned room with a 12 h light-dark cycle. Forty eight male Wistar albino rats were used in this study. Rats were randomly divided into two groups (24 rats per group): untrained (UT) and trained (T) groups. Each group was further subdivided equally into pre- and post-exercise groups (UTpre, UTpost, Tpre and Tpost). At the end of the experiment all animals were scarified by decapitation.Training and acute exhausting exercise protocolsTraining protocol was conducted as describe before [25]. The training program started by swimming sessions that lasted for 5 minutes daily, 5 days per week. The session time was gradually increased over 6 weeks. Ultimately, the rats swam continuously for 30 minutes during the last week of the training program. All rats, trained and untrained, were forced to swim continuously for 120 minutes on the day of the experiment 24 hours after the last training session. Blood samples collectionBlood samples were collected either immediately before or after an acute bout of exhausting swimming exercise. Blood was drawn retro-orbitally from each rat via heparinised microcapillatry tubes. The blood was allowed to coagulate for 30 minutes at room temperature. Blood samples were then centrifuged at 2000 rpm for 10 min to separate serum samples. Serum samples were stored at -20℃. Serum samples were used for the estimation of transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), interleukin 6 (IL-6), interleukin 1 (IL-1β) and tumour necrosis factor alpha (TNF-α). All rats were scarified at the end of experimentation.Biochemical analysisSerum levels of transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), interleukin 6 (IL-6), interleukin 1 beta (IL-1β) and tumour necrosis factor alpha (TNF-α) (Quantikine® ELISA, R&D Systems Inc., MN, USA) were determined by quantitative sandwich enzyme immunoassay technique using an automatic optical reader (SUNRISE Touchscreen, TECHAN, Salzburg, Austria) [26-30].Statistical analysisResults are expressed as mean ± standard error (SE). Student t-test was used for statistical analysis between the same group (UT or T), while repeated-measures Analysis of Variances (ANOVA) was used for statistical analysis of the different groups, using Origin® software and the probability of chance (p values). P values < 0.05 were considered significant.
3. Results
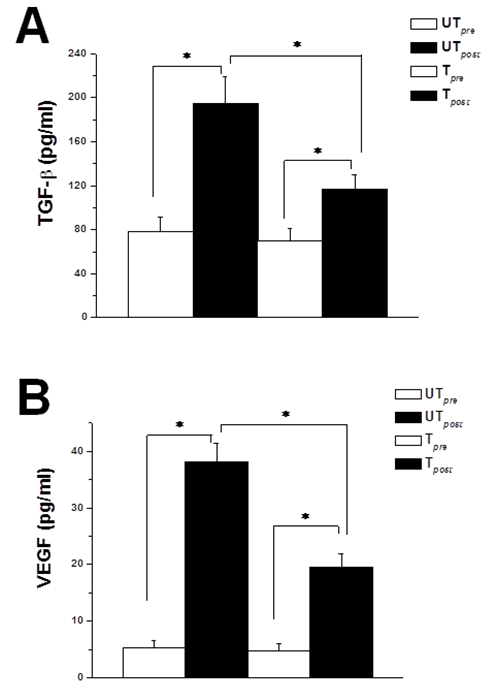
- Serum TGF-β increased significantly following acute bout of strenuous exhausting exercise in both untrained and trained groups when compared to the corresponding baseline levels (Figure 1A). However, there was 2.5 fold increase in serum level of TGF-β in the untrained group (194.5 ± 24.75 (UTpost) vs 78.2 ± 13.57 (UTpre) pg/ml, P < 0.05), while there was 1.5 fold increase in serum level of TGF-β in the trained group (107.3 ± 12.46 (Tpost) vs 69.4 ± 11.82 (Tpre) pg/ml, P < 0.05). Serum TGF-β level was significantly lower in the trained rats when compared to the corresponding values in the untrained group post-exercise (P < 0.05), while it was insignificantly lower in the trained rats when compared to the corresponding values in the untrained rats pre-exercise (P > 0.05).
4. Discussion
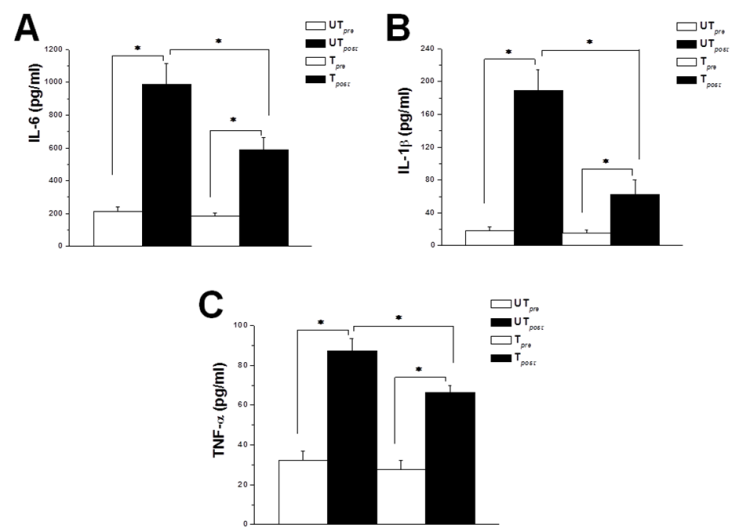
- Physical exercise plays an essential role in improving growth and development in childhood and adolescence. The healthy adaptations to repeated exercise include increased muscle and bone mass, appropriate body fat composition, angio- and arterio-genesi. However, exercise-induced alteration in immune and inflammatory status in response to exercise is similar to those resulting from chronic diseases. Such physiological responses are associated with catabolism rather than anabolism. Here we showed that the effect of exercise on inflammatory cytokines depends on the type, duration and intensity of the physical activity. We also showed that moderate long-term exercise training can modulate the acute exhausting exercise induced rise in circulating growth factors and inflammatory cytokines.In the context of the regulation of myogenesis and promotion of fibrosis, the observed increase in the serum level of TGF-β was of special interest. Physical exercise alters muscle composition and metabolism, a phenomenon referred to as muscle plasticity. Accordingly, since TGF-β influences myogenesis, regeneration, and healing after mechanical strain, it could therefore play an important role in muscle plasticity [31, 32]. During muscle adaptation to physical exercise, an increase in muscle mass -hypertrophy and hyperplasia- is dependent on the intensity and the type of physical training protocol [33]. However, TGF-β was found to increase the degradation of the key regulatory factors of myogenesis MyoD and myogenin in satellite cells. TGF-β has been also shown to suppress the expression of miR-24, a myogenesis promoting factor, and to upregulate myostatin predominantly in the course of terminal differentiation. In these cases TGF-β inhibited muscle growth [34-36]. Our results regarding the serum level of TGF-β can be explained based on the concentration-dependent effects of TGF-β. Moderate regular physical exercise may increase circulating TGF-β to levels that promote myogenesis, and therefore, moderate exercise may endorse the physiological adaptation to muscle exercise. On the other hand, acute exhausting strenuous exercise may cause overproduction of TGF-β. Overproduction of TGF-β could stimulate skeletal muscle fibrosis and inhibit skeletal muscle growth. The cellular response is supposed to meet the changing needs of the muscle tissue during altered environmental conditions. The dynamic changes in the level of TGF-β are therefore crucial for the process of adaptation to physical exercise [6]. The angiogenic growth factor VEGF is an essential facet in angiogenesis. VEGF is probably involved in the vascular remodeling that is caused by exercise and muscle contraction [37]. Our findings regarding the circulating level of VEGF are in agreement with the previous studies that have reported the increase of VEGF after physical exercise in both humans and rats [38-40]. On the contrary, Gustafsson et al. [41] and Gu et al. [42] reported a decrease in the serum level of VEGF after physical exercise, in spite of the upregulation of protein level of VEGF as an early event in muscle adaptation to training. The differences between our study and those, in which the serum level of VEGF decreased after acute bout of exercise, could be caused by the differences in species and exercise protocols. In our study the bout of strenuous physical exercise was longer in duration and much more exhausting. Another possible reason of decreased serum level of VEGF in the aforementioned studies could be the increased binding of VEGF to its endothelial receptors, or an increase in the circulating levels VEGF binding proteins [38].Most cells secrete cytokines; glycosylated polypeptides which influence the cellular functions. Among other pro-inflammatory cytokines, IL-6, and IL-1β and TNF-α initiate and amplify the acute-phase response, stress response and pyrogenesis by modulating immune cell function and migration. Local production of cytokines coordinates the function of innate and adaptive immune cells including growth and differentiation, interaction with vascular endothelial cells and differential expression of cell-surface effectors’ molecules. However, pro-inflammatory cytokines can be released into the circulation in certain pathological states including trauma, sepsis, and thermal injury [43, 44]. Apparently, the body reacts to physical activity as it does during an insult-induced acute subclinical inflammatory response; both pro- and anti-inflammatory cytokines are released into the circulation. Together with other bioactive stress molecules, including glucocorticoids and catecholamines, cytokines regulate various aspects of the immune system [44].In line with the present study, marked increase in the circulating plasma levels of IL-6 after exercise, even without muscle injury, has been consistently reported previously. Exercise induced enhancement in IL-6 gene transcription, up-regulation of IL-6 mRNA, and increase in IL-6 protein expression in contracting skeletal muscle fibers [23, 45, 46]. Eventually, IL-6 was released from skeletal muscles into the plasma during exercise [46]. IL-6 increased in the plasma in an exponential fashion with exercise, and was correlated to the exercise intensity, duration, mass of muscle recruited and subject’s endurance capacity [47]. Our data were also in agreement with other studies which investigated the influence of previous training on plasma level of IL-6. Trained healthy individuals who underwent 3-hour session of dynamic two-legged knee-extensor exercise at 50% of their individual maximal power output demonstrated 20-fold increase in plasma-IL-6, 16-fold increase in IL-6 mRNA, , and marked IL-6 release from working muscle. However, when the same exercise protocol was applied in healthy untrained subjects, even higher levels of IL-6 were detected [48, 49]. Although several reports shows that strenuous exercise induces elevations in IL-1β and TNF-α, yet results from different studies were inconsistent. This could possibly be in part due to differences in the experimental design, time of blood sampling, and IL-1β and TNF-α assay sensitivity [50, 51]. Exhausting exercise can increase plasma levels of IL-1β and TNF-α by augmenting blood level of endotoxin, most likely through an increase in gastrointestinal permeability. Mild exercise-induced endotoxemia triggers primary and secondary lymphoid tissues resulting in the production and release of IL-1β and TNF-α in the circulation [52].
5. Conclusions
- The results of the present study suggest that moderate voluntary regular exercise training can modulate the generation of exercise-induced generation of TGF-β, VEGF, IL-6, IL-1β and TNF-α, which was evident by the attenuation of the rise in growth factors and inflammatory cytokines following an acute bout of exhausting physical exercise. Therefore, long-term moderate exercise might be effective in the prevention of inflammation cased be muscular activities. Our findings may also help in better understanding of muscle plasticity phenomenon, and how muscle adapts to physical exercise. The clinical implications of the present study are of great importance, especially in the context of type, duration and endurance of exercise. We showed here that some strenuous exercise programs may have potentially deleterious effects, which could be prevented by prior moderate training. For example, given that exercise is one of the gold standard treatments for heart failure, it is therefore very important to understand the mechanism and impact of physical exercise on the production and release of growth factors and inflammatory cytokines into the circulation.
AKNOWLEGMENTS
- Authors wish to thank the Faculty of Medicine - Menoufia University for providing all required facilities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML