-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2014; 4(6): 203-205
doi:10.5923/j.ajmms.20140406.01
Acute Kidney Injury Following Basal Ganglia Haemorrhagic Stroke – Is Apathy the Predisposing Factor?
Norhamizan Hamzah, Norhaina Mahli, Mazlina Mazlan
Department of Rehabilitation Medicine, Faculty of Medicine, University Malaya, Kuala Lumpur, Malaysia
Correspondence to: Norhamizan Hamzah, Department of Rehabilitation Medicine, Faculty of Medicine, University Malaya, Kuala Lumpur, Malaysia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Apathy, which is defined as a lack of motivation not attributable to delirium, dementia, or emotional distress, is a clinical feature following stroke. It is reported to be present early after a stroke but can occur up to six months albeit at lower percentages. In this case series, we presented two stroke cases with large basal ganglia haemorrhages who developed prominent symptoms of apathy within the first two weeks after stroke. We confirmed the presence of apathy in both patients using the Apathy Evaluation Scale–Clinician Version (AES-C). Since depression had some overlapping symptoms with apathy, we also confirmed the absence of depression using the Hospital Anxiety and Depression Scale (HADS). Both patients showed poor inpatient rehabilitation progress and subsequently developed acute kidney injury, believed to be the result of the apathy.
Keywords: Basal ganglia haemorrhage, Apathy, Acute kidney injury, Rehabilitation
Cite this paper: Norhamizan Hamzah, Norhaina Mahli, Mazlina Mazlan, Acute Kidney Injury Following Basal Ganglia Haemorrhagic Stroke – Is Apathy the Predisposing Factor?, American Journal of Medicine and Medical Sciences, Vol. 4 No. 6, 2014, pp. 203-205. doi: 10.5923/j.ajmms.20140406.01.
1. Introduction
- The prevalence of post-stroke apathy ranges from 15% to 35% and it is more common within the first two weeks [1-3]. Acute intracerebral haemorrhage and basal ganglia lesions were among the predictive factors reported for apathy post stroke [1,4]. Syndromes of apathy include lack of motivation, diminished goal directed cognition and diminished emotional concomitants of goal–directed behaviour [5]. The manifestation of such behaviours may lead to or hasten the development of other acute complications following stroke. Whilst medical complications such as infections and falls are easy to specify and frequently reported [6, 7], complications relating to cognition such as apathy were reported less frequently. In this case report, we present two patients; both with large basal ganglia haemorrhages who developed acute kidney injury (AKI) and subsequently resulted in poor inpatient rehabilitation progress. Symptoms of apathy are believed to contribute to the development of AKI in both patients.
2. Case One
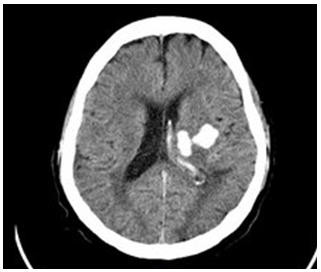
- A 57 years old man with uncontrolled hypertension presented with right-sided body weakness, slurred speech and facial asymmetry. His Glasgow Coma Scale (GCS) score on hospital arrival was 15/15 with blood pressure reading of 230/120 mmHg. An initial CT scan of the brain showed an intracranial haemorrhage involving the left basal ganglia, left thalamic and left external capsule with extension of bleed into left lateral ventricle and the presence of perilesionaloedema (Figure 1). There was no seizure reported and he was managed conservatively.
3. Case Two
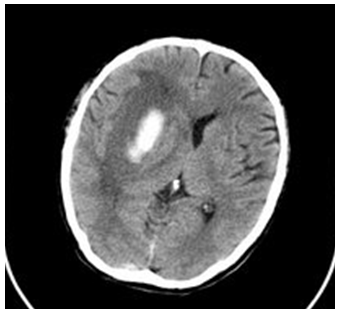
- A 58 years old man, with longstanding hypertension, presented with sudden onset of left sided weakness and facial asymmetry. His GCS score was 15/15 on arrival at the hospital. The CT brain scan reported a right basal ganglia haemorrhage with extensive mass effect involving compression of ipsilateral lateral ventricle and dilatation of contralateral ventricle and the presence of midline shift of 0.4 cm to the left (figure 2). He was treated conservatively. On day four post stroke, the patient was referred for rehabilitation. On evaluation, he was able to obey a two-step command consistently and MMSE score of 21/30.
4. Discussion
- The occurrence of apathy post stroke has been shown to be associated with reduced cognitive function, increased disability and poor rehabilitation outcome [3,8].However, the association of apathy post stroke with a serious medical adverse outcome such as AKI is rarely reported. Our two patients shared features of apathy during their inpatient rehabilitation stay, which subsequently led to poor oral intake and dehydration. This in turn led to AKI, which halted the functional recovery and rehabilitation progress.Misclassification of apathetic patients as being depressed is not uncommon, especially when apathetic patients have a two-fold risk of concurrent post stroke depression [3, 9]. In our patients, the lack of dysphoria and other signs of depression such as decreased sleep, self-blame or suicidal thoughts have led us to suspect presence of apathy early. There is no consensus on diagnostic criteria for apathy as a syndrome. Different methods are used to assess apathy and the findings may vary if the assessment is informant-based, patient-based or clinician-based [3]. We chose to use the clinician-based instrument, AES-C, in both patients since it is a reliable and valid measure for the characterization and quantification of apathy [10]. Identification of apathy was based on the high score of AES-C and the normal score of HADS. In this report, diagnosis of apathy was made early within the first two weeks post stroke. The rehabilitation program was adapted to the patient’s needs and non-pharmacological approach was decided as an initial management. The patient’s family members were included in the program to improve patient’s participation in therapy. We did not initiate any pharmacological use since apathy was recently diagnosed and there is insufficient evidence to support a pharmacological approach [3]. Previous studies have reported clinical benefits of psychostimulants to occur within the first 30 days of apathy and we were still within the window period [11]. However, both patients deteriorated rapidly due to dehydration before any improvement in apathy was observed. Since stroke patients are at risk of developing apathy, it should routinely be ruled out in all patients, particularly in those with basal ganglia lesions. Most studies have shown that apathy post stroke affects the rehabilitation process in general. However, we also demonstrated that in the presence of apathy, the risk of medical complications may be higher and faster than estimated as shown in our patients. Heightened awareness of the possible complications from apathy during the initial rehabilitation period may lead to earlier recognition and management.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML