-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2014; 4(5): 192-202
doi:10.5923/j.ajmms.20140405.10
Swimming Exercise Ameliorates Elevated Blood Pressure and Vascular Endothelial Dysfunction in Old Rats
Nermin Rezk, Safaa M. Elkotb, Yahya M. Naguib
Clinical Physiology Department, Faculty of Medicine, Menoufia University, Menoufia, Egypt
Correspondence to: Yahya M. Naguib, Clinical Physiology Department, Faculty of Medicine, Menoufia University, Menoufia, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Background and Aim: Advancing age increases the risk of cardiovascular diseases, resulting in higher rates of morbidity and mortality. We tested the hypothesis that habitual swimming exercise would reverse age-related systolic hypertension, vascular dysfunction, hyperactivity of sympathetic nervous system, dyslipidemia, and oxidative stress. Methods: Thirty male Wistar albino rats were used in the present study. Rats were divided (10/group) into young (3-4 months), old sedentary (22-24 months), and old exercised (6 weeks swimming protocol) rats. Systolic blood pressure (SBP), endothelial function, sensitivity of sympathetic nervous system, and renal artery Doppler flowmetry were evaluated. Serum lipids, renal biomarkers and oxidant-antioxidant status were assessed.Results: SBP was significantly higher (100 ± 4.83 vs 80 ± 3.34 mmHg) in old sedentary when compared to young rats, and was associated with reduced endothelial-dependent dilation (EDD), increased sympathetic adrenergic vasoconstriction, dyslipidemia, reduced renal artery blood flow velocity (RBFV), and increased renal artery resistance (RAR). Swimming exercise restored SBP, EDD, sympathetic adrenergic vasoconstriction, serum lipids, RBFV, RAR, to levels almost comparable to young rats. Swimming increased significantly total antioxidant capacity in old exercised rats (2.37 ± 0.33 vs. 1.92 ± 0.44 mM /L), while serum malondialdhyde was decreased (2.58 ± 0.23 vs. 4.42 ± 0.14 ηM/L), when compared to old sedentary rats. All results were statistically significant (p < 0.05).Conclusion: We report here that habitual swimming ameliorates age-dependent systolic hypertension and endothelial dysfunction possibly by anti-oxidant mechanism.
Keywords: Aging, Isolated systolic hypertension, Endothelial dysfunction, Sympathetic hyperactivity, Dyslipidemia, Antioxidants
Cite this paper: Nermin Rezk, Safaa M. Elkotb, Yahya M. Naguib, Swimming Exercise Ameliorates Elevated Blood Pressure and Vascular Endothelial Dysfunction in Old Rats, American Journal of Medicine and Medical Sciences, Vol. 4 No. 5, 2014, pp. 192-202. doi: 10.5923/j.ajmms.20140405.10.
Article Outline
1. Introduction
- Aging, or senescence, is the primary risk factor for cardiovascular diseases (CVD) and the later are the leading cause of mortality worldwide [1, 2]. Senescence is an independent factor associated with vascular aging even in the absence of other cardiovascular risk factors [3]. Vascular aging is characterized by structural (large elastic artery stiffening) and functional (endothelial dysfunction) alterations of the vascular endothelium and smooth muscle cells [4-6]. Aged-induced large elastic artery stiffness is mediated in part by increased collagen I deposition, reduced elastin and protein modifications by advanced glycation end-products (AGEs) [4, 7]. Endothelial dysfunction is a progressive phenomenon starting in the middle age, and it is considered as one of the main mechanisms underlying the development of CVD and atherosclerosis in aging [8]. Age-dependent endothelial dysfunction, indicated by the impairment of endothelium-dependent dilation (EDD), is a consequence of reduced nitric oxide (NO) bioavailability [9-11]. Endothelial dysfunction is associated with the major causes of morbidity and mortality, therefore, maintaining its optimal function is thought to be a crucial determinant of healthy aging [12]. Evidences demonstrating the presence of endothelial dysfunction in aging have been reported in animal models as well as in humans. The reduction in EDD in particular has been consistently demonstrated in different parts of the vasculature from aged animals and humans [3, 13-17]. The reported data show that the pathogenesis of the age-dependent endothelial dysfunction is clearly a multifactorial process [8]. Thus, approaches aimed to preserve or improve the endothelial function would be fundamental for the prevention of CVD in aging. Oxidative stress has been implicated in the genesis of age-dependent vascular dysfunction as well as in several diseases including hypertension and diabetes [18-21]. Reactive oxygen species (ROS) are usually produced into the vascular wall in a controlled manner. Physiological ROS function as cell signalling initiators. The naturally occurring antioxidant defence mechanisms, both enzymatic (superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase), and non-enzymatic (vitamin C, vitamin E, uric acid), normally inactivate ROS and repair any resultant damaged tissue [22, 23]. However, under pathological conditions, increased ROS levels lead to endothelial dysfunction and inflammation. Several systems are proposed to be the sources for the increase ROS production in the vascular tissue, such as NADPH oxidase, xanthine oxidase, uncoupled NO synthase, and the mitochondrial respiratory chain [21, 24]. Overproduction of ROS, especially the superoxide anion (O2-•), accelerates the degradation of NO resulting in the formation of the peroxynitrite anion ONOO (reactive nitrogen species, RNS). Credible data were reported showing a substantially enhanced age-induced formation of ONOO− in the vasculature of both aged humans and animals [25, 26]. Unlike O2-•, ONOO− easily penetrates the cell membrane causing oxidative modifications of lipids, proteins, and DNA [27]. Accumulating epidemiological, clinical and experimental evidences show that exercise training can substantially influence age-dependent cardiovascular dysfunction and reduce CVD risk. Habitual exercise has been suggested to improve blood pressure, endothelial function, arterial wall elasticity, as well as amelioration of the inflammatory process, and oxidative stress [28, 29]. In view of that, the aim of the present study was to evaluate the effect of regular swimming exercise on age-dependent vascular dysfunction. To do so, we tested the hypothesis that SBP, conduit artery EDD but not endothelium-independent dilation, vascular adrenergic system, aging-induced dyslipidemia and renal dysfunction can be influenced by habitual swimming exercise. We then proposed that the improvement of aforementioned phenomena by habitual exercise can be explained by an antioxidant mechanism.
2. Materials and Methods
- AnimalsThe experimental procedures were conducted in adherence to the Guiding Principles in the Use and Care of Animals published by the National Institutes of Health (NIH Publication No 85–23, Revised 1996). Animal care and use was approved by the University Ethics Committee. Animals were kept for 10 days prior to the start of the study to allow proper acclimatization. The animals were fed standard laboratory chow and allowed free access to water in an air-conditioned room with a 12 h light-dark cycle. The experiments were performed in male Wistar albino rats in three groups (10 rats per group): young (3–4 months old, weight 150- 200 g), old (22–24 months old, weight 400–500 g), and old exercised (22–24 months old, weight 400–500 g) groups.Swimming protocolSwimming protocol was conducted as describe before [30]. The training program started by swimming sessions that lasted for 5 minutes daily, 5 days per week. The session time was gradually increased over 6 weeks. Ultimately, the rats swam continuously for 30 minutes during the last week of the training program. Measurement of systolic blood pressureSystolic blood pressure was determined in the rats by means of a rat-tail pressure detecting equipment (Harvard apparatus Ltd, Aden Berge, England) connected to a pneumatic transducer (Harvard U.K.). Changes in pressure were recorded via a physiograph (MK III-S, Narco BioSystem, USA).Assessment of endothelial functionEndothelial function was determined by comparing the vasorelaxation response to acetylcholine (ACh), an endothelium-dependent vasodilator, with that of sodium nitroprusside (SNP), an endothelium-independent vasodilator. Briefly, thoracic aortic rings were mounted onto hooks, suspended in organ chambers filled with Krebs Hanseliet buffered solution (mM/L: NaCl 118.3, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25.0, glucose 11.0, pH=7.4), aerated with 95% O2 and 5% CO2 at 37°C, and connected to force displacement transducers (Grass, USA) to record changes in tension via a physiograph (MK III-S, Narco BioSystem, USA). After equilibration for 60 min at a preload of 1 g, the rings were precontracted with norepinephrine (NE, 10 μM). Once a stable contraction was achieved, the rings were exposed to 1 μM ACh. After the response was stabilized, the rings were washed and allowed to equilibrate to baseline. The procedure was then repeated with an endothelium independent vasodilator (1 μM SNP) to determine smooth muscle function. Endothelial-dependent vasodilator dysfunction was defined as a reduced vasorelaxation in response to ACh with a normal response to SNP.Biochemical analysisSerum urea and creatinine levels (ELITech, France) were determined by routine kinetic and fixed rate colorimetric methods on a Jenway Genova autoanalyser (UK). Serum total antioxidant capacity (TAC) and malondialdehyde (MDA) (QuantiChrom™, BioAssay Systems, USA) were determined by quantitative colorimetric method.Assessment of renal hemodynamicsMeasurement of renal blood flow velocity (RBFV) and renal artery resistance (RAR) was done using pulsed Doppler flowmeter (Hadeco, Hayashi Denki Co. Ltd., Japan) as described previously [31]. Briefly, rats were anesthetized with 25% urethane (0.6 ml/100gm, intra-peritoneal injection), and a midline abdominal incision was made exposing the left renal artery. After the tip of the probe was filled with coupling gel, the probe was placed over the blood vessel until a stable record could be achieved. Haematoxylin and Eosin (Hx & E) stainSpecimen from the aortas were taken and fixed in 10% formol saline for 5-7 days. The specimens were washed in tap water for 10 minutes dehydrated in graded ethanol solutions (70%, 90% over night and 100% ethanol solution for three changes one hour each). The specimens were then cleared in xylene for 20-30 times according to the size of the specimen (guided by inspection at five minutes intervals). The specimens were impregnated in soft paraffin wax at 55-60 °C for two hours then in hard paraffin wax at room temperature in moulds. Finally tissue blocks were cut into section of five microns thickness by using rotator microtome. Tissue sections were dipped in a warm water-bath, picked up on clean slides, and placed on hot plate for two minutes. Finally, tissue sections were stained with haematoxylin and eosin stain for general architecture of the studied tissues.Statistical analysisResults are expressed as mean ± standard error (SE). Student t-test or repeated-measures Analysis of Variances (ANOVA) were used for statistical analysis of the different groups whichever appropriate, using Origin® software and the probability of chance (p values). P values < 0.05 were considered significant.
3. Results
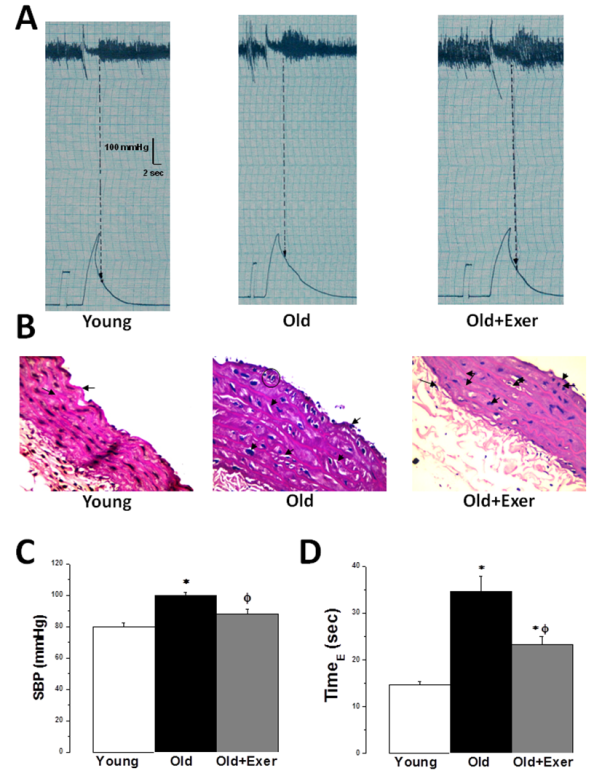
- Systolic blood pressure (SBP) increased significantly in the old sedentary rats when compared to the young rats group (100 ± 4.83 vs 80 ± 3.34 mmHg, P < 0.05). SBP decreased significantly in the old exercised rats when compared to the old sedentary group (88.33 ± 5.07 mmHg, P < 0.05). There was no statistically significant difference in SBP between young and old exercised groups (P > 0.05) (figures 1A and 1C). The time interval between the cessation of the pulse wave and reappearance of full pulsation (referred to as TimeE, figures 1A and 1D) was significantly longer in the old sedentary rats when compared to the young rats group (34.67 ± 3.53 vs 14 ± 1.09 seconds, P < 0.05). TimeE decreased significantly in the old exercised rats when compared to the old sedentary group (24.67 ± 1.76 seconds, P < 0.05). However, TimeE was still significantly longer in old exercised rats when compared to young rats (P < 0.05).
4. Discussion
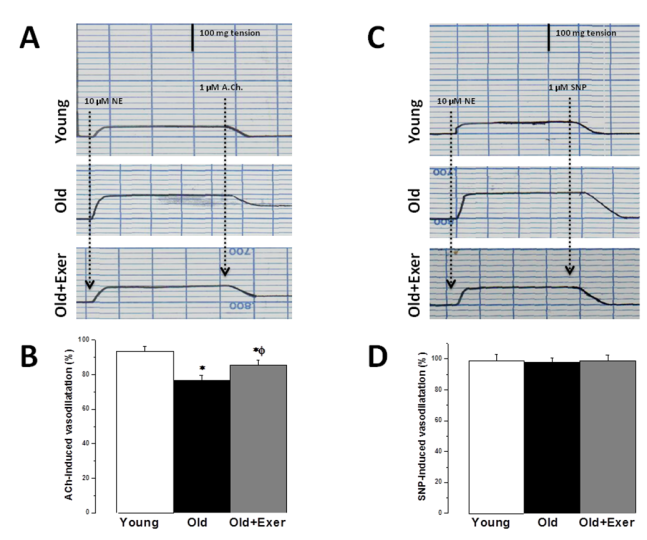
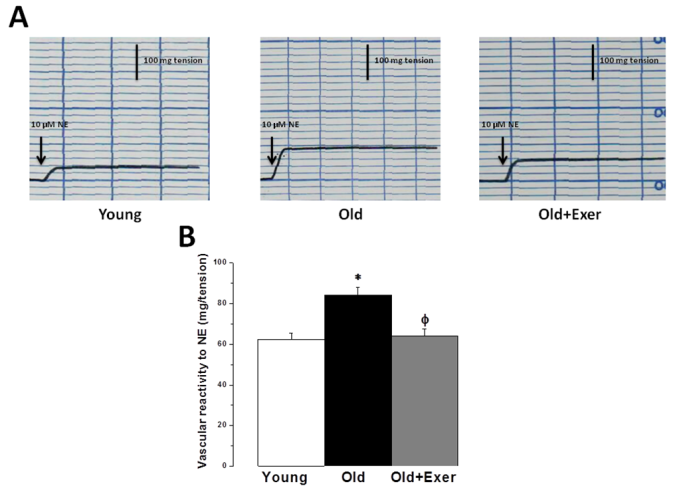
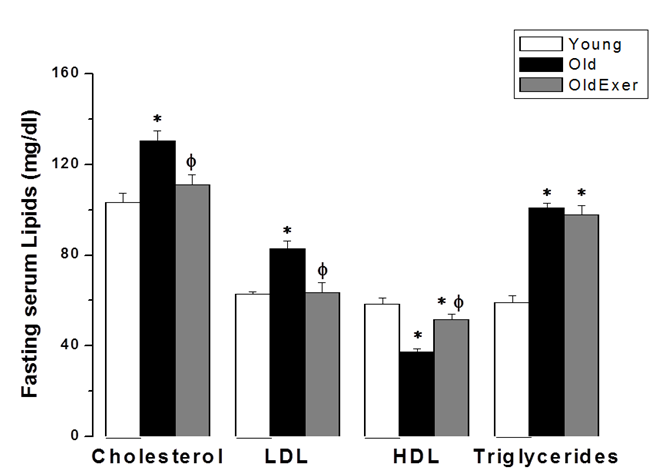
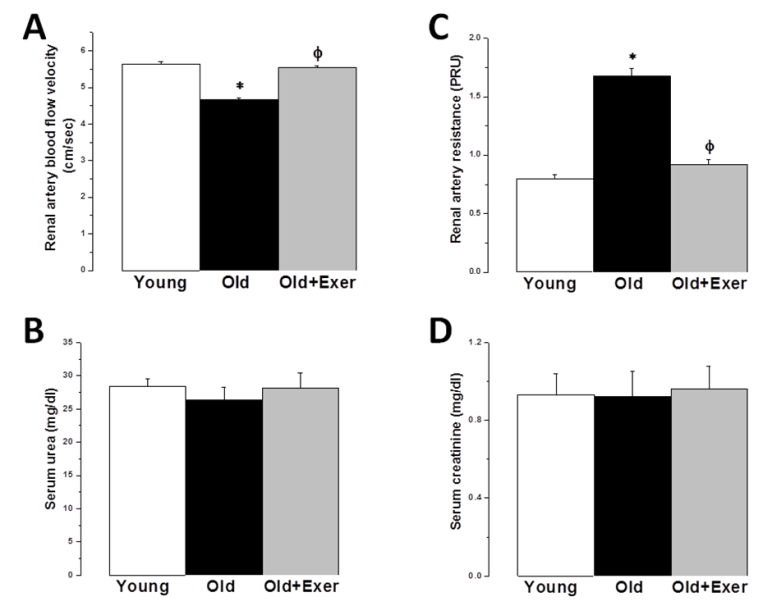
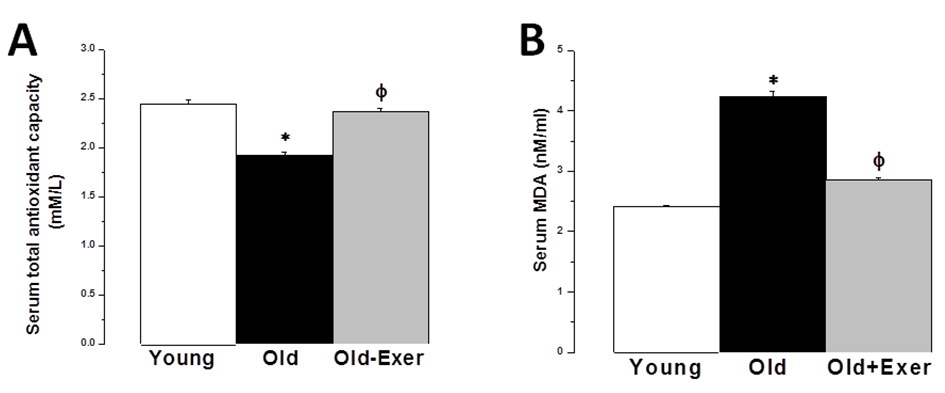
- Aging is the main risk factor for CVD. The increase in CVD risk with aging can be generally attributed to vascular endothelial dysfunction and large elastic artery stiffening [32, 33]. In the present preclinical study, we suggest that regular swimming exercise has a potential role in the prevention of age-dependent CVD. The present study also provides an insight into the mechanisms by which aerobic exercise may reverse arterial aging, which was evidenced by restoring age-dependent endothelial dysfunction, altered sympathetic nervous system (SNS) activity, dyslipidemia, renal dysfunction and oxidative stress. We proposed that exercise-induced improvement of age-dependent alterations can be explained by antioxidant mechanism. In the present study we showed that systolic blood pressure was significantly increased in old rats, and that habitual swimming exercise could protect against age-related hypertension. Isolated systolic hypertension (ISH) is accounted for the majority of hypertension in the elderly. ISH is associated with serious adverse outcomes, including stroke, CVD, and death. ISH, as well as diastolic heart failure and small vessel disease, is a consequence of arterial aging [34]. We analyzed the time between the cessation of pulse waves and the recovery of full pulsations (TimeE, figure 1). This time window is clearly dependent on, and therefore, can be used to assess arterial elasticity. We demonstrated that age induced alterations in arterial elasticity can be improved by habitual exercise. In response to normal aging, structural and functional changes occur in the vasculature most prominently in the proximal aorta. Increased arterial stiffness, as a result of increased intima-media thickness and accumulation of collagen produced by invading vascular smooth muscle cells, results in a raise in aortic pulse wave velocity. This leads to an early return of the reflected pressure wave and thus, elevation in systolic pressure. Moreover, the decrease in aortic elasticity also leads to greater peripheral runoff during systole [20].Specimens from the thoracic aorta demonstrated marked age-induced structural changes involving all layers of the arterial wall. These changes were in the form of endothelial ulceration, vacuolization and lymphocytic infiltration of subendothelial tissue. The media and adventitia showed marked attenuation, branching and breaking of elastic and collagen fibers. These findings were in agreement with the previously published investigations [4]. Large elastic arteries stiffness, e.g. aorta and carotid arteries, progressively increases with advancing age even in healthy individuals and animals, resulting in lower arterial compliance. On the contrary, peripheral arteries in healthy humans do not normally stiffen [35-37]. The reduction in arterial wall elasticity contributes to the development of systolic hypertension with aging even in the absence of evident pathological arteriosclerosis in humans and animals. Increased stiffness reduces the reservoir function of conduit arteries near the heart with subsequent increase in pulse wave velocity, both of which elevates systolic as well as pulse pressures [38]. The Baltimore Longitudinal Study of Aging provided the initial clue that habitual aerobic exercise might attenuate age-associated increases in large elastic artery stiffness [39]. We proved in the present investigation that regular swimming exercise attenuated age-induced increase in elastic artery stiffness, which led to improvement of arterial compliance with a resultant significant decrease in SBP. However, this was not accompanied by any histopathological evidence that swimming reversed or even minimized age-related structural change in the arterial wall in old exercised rats (figure 1). It has been reported before that reduced elastic arteries stiffness in trained old rodents is independent of structural changes in the arterial wall [40, 41], and to our knowledge there are no available data supporting such possibility.Consistent with previously published reports [29, 42-44], the present study showed that vascular endothelial dysfunction develops with aging, which was evident by impairment of EDD but not endothelial dependent dilatation (figure 2A). EDD is mediated by reduced NO bioavailability [45]. Reduced NO production results of NADPH oxidase-associated increases in superoxide and inadequate BH4 bioactivity [43]. We showed here that 6 weeks of swimming exercise reversed the impaired EDD in old rats to levels observed in young control rats. Importantly, our findings showed that in the presence of a NO donor (SNP), arterial dilatation was completely preserved. Together, our data are consistent with the possibility that swimming exercise ameliorated vascular endothelial dysfunction in old rats and restored EDD apparently by, at least in part, restoring NO bioavailability. Voluntary wheel running has been shown to restore endothelial function and EDD in conduit arteries in old mice by preservation of NO bioavailability [42].Aging causes morphological and functional changes in both cardiovascular and autonomic nervous systems (ANS). The ANS changes involve increased sympathetic and decreased parasympathetic activities, with higher incidence of cardiovascular diseases and hypertension [46, 47]. Regular aerobic exercise has been shown to decrease elevated SNS activity in aging vasculature, heart failure and hypertension [30]. Accordingly, we examined the effect of swimming exercise on the vascular reactivity to norepinephrine (NE). We showed that swimming exercise significantly reduced vascular reactivity to NE in old exercised rats when compared to corresponding value in old cage restricted sedentary rats (figure 3). Our results were in agreement with previously published data suggesting that regular exercise reduce the vascular wall sensitivity to NE in an endothelial-dependent manner [48, 49]. The effect of swimming exercise on enhanced sympathetic-mediated vasoconstriction in old rats can be explained by two mechanisms. The first possibility is that swimming exercise may have elicited adaptations in the adrenergic system. Since the SNS was activated during each bout of exercise, repeated activation of SNS may have resulted in an attenuation of sympathetic activity [47, 50]. The second possibility is that swimming, by restoring NO bioavailability, may have allowed NO to decrease the overall sympathetic excitability within the brainstem possibly by acting through higher brain regions [51, 52].Many risk factors predispose elderly people to develop pathologies related to failure of the heart and vasculature, including age-induced dyslipidemia. During aging, hepatic lipid modifications occur. Interestingly, age-related activation of the rate-limiting enzyme 3-hydroxy-3- methylglutaryl coenzyme A reductase (HMGR) which is responsible for the de novo synthesis of cholesterol has been associated with increased levels of ROS. Studies of 24 month old male rats showed increased plasma cholesterol levels and increased hepatic cholesterol synthesis accompanied by full activation of HMGR, which was dependent on the well-known age-related increase in ROS [53-55]. We have shown that swimming exercise significantly decreased age-dependent elevated cholesterol and LDL levels in old exercised rats when compared to the sedentary controls (figure 4). Swimming exercise also increased levels of HDL in aged rats. Taken our data together, it may be postulated that the antioxidant effect of aerobic exercise decreased HMGR activity with subsequent restoration of normal hepatic lipid modification. However triglycerides level was insignificantly altered between all study groups, which was in agreement with previously published data [56-58].Hypercholesterolemia is considered a factor that contributes to cardiovascular diseases, and also, renal dysfunction [59]. It was necessary then to investigate whether the kidneys were also affected by aging and exercise. Apparently, serum biomarkers showed that the kidneys were not critically insulted by the aging processes. However, we showed here that renal artery resistance was increased and renal blood flow velocity was decreased in aged sedentary rats (figure 5). Activation of the rennin-angiotensin- aldosterone system (RAAS) is associated with the development of endothelial dysfunction and arterial stiffness [60-62]. Activation of RAAS results in the production of angiotensin II, the later contributes to cardiovascular remodelling and regulation of blood pressure [63]. Interestingly, angiotensin II has been linked to the development of vascular aging through oxidative stress [64]. In the present study, swimming exercise decreased renal artery resistance and improved blood flow in old rats. Based on our data, this could be explained by the ability of aerobic exercise to restore NO bioavailability and enhancement of EDD in renal artery. The present investigation showed that aging caused significant decrease in serum TAC, and simultaneously increased serum level of MDA. It has been proposed that aging may attenuate the antioxidant defence mechanisms, and therefore, may cause a decrease in TAC [28]. Aging-induced oxidative stress, particularly arterial oxidative stress, has been well studied and several reports have demonstrated that increased arterial oxidative stress in aging is associated with increased superoxide production and reduced activity of antioxidant enzymes such as superoxide dismutase (SOD) [10, 11]. Aging can increase the production of superoxide anion (∙O2−). ∙O2− itself is chemically inert; however, when it combines with NO at a diffusion limited rate, it becomes a highly reactive species, peroxynitrite (ONOO−). ONOO− can initiate both nitrative and oxidative reactions with proteins, lipids, and DNA. A characteristic reaction of ONOO− is the nitration of protein bound tyrosine residues to produce nitrotyrosine, and the production of nitrotyrosine has been used extensively as a footprint for ONOO− [65]. MDA is a stable metabolite of the ROS-mediated lipid peroxidation cascade, often used as index of oxidative stress [66]. The increased production of oxidants with concurrent exhaustion of the antioxidant capacity diminishes the availability of NO, with subsequent endothelial dysfunction and impaired EDD [67]. We showed here that the serum TAC in old exercised rats was significantly increased, while serum MDA level was decreased when compared to old sedentary controls (figure 5A). These results implied that the alteration of oxidant-antioxidant balance in old rats was somehow parallel with the vascular endothelial dysfunction. Previously published results supported our findings; regular swimming attenuated age-induced oxidative stress and increase antioxidant levels shifting the cellular milieu to a less oxidative state, suggesting a preventive role for aerobic habitual exercise in various tissues including skeletal muscle, liver and brain [68-71]. Swimming exercise-induced reduction in oxidative stress and elevation of TAC may underlie the improvement in age-related vascular alterations.
5. Conclusions
- We showed here that, at least in part, oxidative stress may be the central underlying mechanism for age-induced hemodynamic alterations. Habitual aerobic exercise can play an important role in attenuating age-related isolated systolic hypertension and vascular dysfunction, and thus decreasing the risk of CVD. Oxidative stress can be considered a potential therapeutic target in aging population.
AKNOWLEGMENTS
- Authors wish to thank Menoufia University for providing most of the required facilities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML