-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2014; 4(5): 168-174
doi:10.5923/j.ajmms.20140405.06
Oxidative Stress in Symptomatic Malaria Parasitemic Pregnant Women from Malaria Endemic Area of Nigeria
Ogbodo S. O.1, Okaka A. N. C.2, Nwagha U. I.3, Ejezie F. E.4, Okafor C. S.2
1Dept. of Chemical Pathology, Goldlife Medical Laboratories, Enugu, Nigeria
2Dept. of Applied Biochemistry, Faculty of Biosciences, Nnamdi Azikiwe University, Awka, Nigeria
3Dept. of Physiology and Obstetrics/Gynaecology, University of Nigeria, Enugu Campus, Nigeria
4Dept. of Medical Biochemistry, University of Nigeria, Enugu Campus, Nigeria
Correspondence to: Ogbodo S. O., Dept. of Chemical Pathology, Goldlife Medical Laboratories, Enugu, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
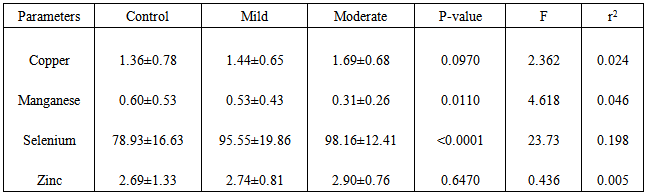
During pregnancy, a lot of stresses are experienced, caused by decrease in macro and micronutrients, changes in plasma volume, tissue hypoxia and disease conditions like malaria, helminthiasis, HIV, diabetes, hypertension etc. Earlier reports indicate that asymptomatic malaria parasitemia does not induce additional oxidative stress during pregnancy.This study aims to find whether symptomatic malaria parasitemia causes additional oxidative stress on pregnant women.It is a cross-sectional study involving 119 febrile pregnant women attending antenatal clinics of some hospitals in a malaria endemic town of Enugu, Enugu State of Nigeria. They were aged between 24 and 36 years, and mainly in their second (14 – 28 weeks) and third (29 weeks and above) trimesters of pregnancy. Malaria density was estimated by absolute malaria count, while antioxidant minerals – copper, manganese, selenium and zinc were determined using atomic absorption spectrophotometer.Results obtained for negative, mild and moderate malaria densities were 1.36±0.78mg/dL, 1.44±0.65mg/dL and 1.69±0.68mg/dL for copper; 0.60±0.53mg/dL 0.53±0.43mg/dL and 0.31±0.26mg/dL for manganese; 78.93±16.63µg/dL, 95.55±19.86µg/dL and 98.16±12.41µg/dL for selenium; and 2.69±1.33mg/dL, 2.74±0.81mg/dL and 2.90±0.76mg/dL for zinc. Analysis of variance indicates that copper and zinc showed no significant changes as malaria density increased (p=0.0970 and 0.6470 respectively) while manganese and selenium showed significant changes (p=0.0110 and <0.0001 respectively). Though correlations between malaria density and the levels of these antioxidants were relative and non-significant, copper, selenium and zinc showed positive relation while manganese showed negative relation.We are of the opinion that symptomatic malaria parasitemia induces extra oxidative stress on pregnant women. This calls for simultaneous determination of relevant antioxidants in suspected malaria parasitemia to help in better treatment and management of the disease, especially during pregnancy.
Keywords: Oxidative stress, Malaria parasitemia, Pregnancy
Cite this paper: Ogbodo S. O., Okaka A. N. C., Nwagha U. I., Ejezie F. E., Okafor C. S., Oxidative Stress in Symptomatic Malaria Parasitemic Pregnant Women from Malaria Endemic Area of Nigeria, American Journal of Medicine and Medical Sciences, Vol. 4 No. 5, 2014, pp. 168-174. doi: 10.5923/j.ajmms.20140405.06.
Article Outline
1. Introduction
- Malaria parasitization is thought to increase oxidative stress, and therefore reactive oxygen species (ROS) in patients [1]. Hence the virulence of malaria parasites and prognosis of the disease seem to depend largely on the patients’ antioxidant capacities, which in turn is determined by the concentrations of antioxidant micronutrients. These antioxidants play significant immuno-modulatory roles in health and disease, including malaria infection. Therefore, antioxidant micronutrients levels in malaria infected patients are possible determinants of the virulence of the parasites, extent of oxidative stress and prognosis of the disease. During pregnancy, a lot of stress is experienced physiologically and pathologically. The physiological stress is due to changes resulting from increased demands for nutrients, and changes in plasma volume. These increased demands eventually lead to decrease in micronutrients (especially antioxidant micronutrients) like vitamins and minerals, macronutrients particularly serum proteins, and ultimately haemoglobin levels [2-5]. In normal pregnancy, the earliest stages of development take place in a low oxygen environment [6] – tissue hypoxia. Tissue hypoxia is known to promote release of ROS that are potentially damaging to the cardiovascular system [7]. However, physiological hypoxia of early gestational sac is beneficial because it protects developing fetus against deleterious and teratogenic effects of ROS [6]. Pathological stress is mainly due to disease conditions including malaria, helminthiasis, HIV, diabetes and hypertension. Pregnant women, especially primigravidae and secundigravidae, are known to have low immune status, hence they are easily prone to malaria attack [8]. This is more pronounced in our rural areas burdened by poor socio-economic conditions and complicated by unhealthy and filthy environments that encourage and increase these infections. Moreover, in response to malaria and other infections, phagocytic cells such as polymorphonuclear leucocytes and macrophages usually engage in respiratory burst as a host cell-mediated immune response, yielding free radicals that react to yield ROS [1], [9, 10]. All these conditions generate enormous oxidative stress implicated in many perinatal and maternal illnesses like abortion, preeclampsia, stillbirth, low birth weight and even death [11-15].Our environment is known to have high percentage of malaria parasitemia in pregnancy [4, 16]. Many changes have also been reported in the serum levels of some nutritional parameters (including antioxidants) in these parasitemic pregnant women, and even children [5, 17], including uncomplicated pregnancies [18, 19]. But our earlier report indicated that asymptomatic malaria parasitemia does not induce additional oxidative stress during pregnancy [20]. However, physical and clinical conditions of asymptomatic parasitemic patients are not the same with those of symptomatic patients, hence it is possible that their physiological and biochemical conditions will not be the same. We attempted to determine the extent of these biochemical differences, as they affect oxidation, in symptomatic parasitemic pregnant women. For this, we determined the concentrations of some antioxidant minerals (copper, manganese, selenium and zinc) in symptomatic parasitemic pregnant women.
2. Materials and Methods
2.1. Ethical Clearance
- Ethical clearance for this study was obtained from the Research Ethics Committee of University of Nigeria Teaching Hospital, Enugu, while additional consents of the patients were sought and obtained after counseling. The patients also got access to their results for treatment without paying for them.
2.2. Patients
- This is a cross-sectional study and patients were drawn from the antenatal clinics of some hospitals in Enugu metropolis known to have high prevalence of malaria in pregnant women [4, 16]. A total of 119 pregnant women who already have symptoms of malaria like fever, headache and unexplained weakness, and who have not taken any anti-malaria drugs since the onset of the condition(s) were recruited and enlisted for the study as they come for their antenatal visits. Out of these symptomatic women, 51(42.9%) had mild malaria density (1 – 999/µl or +), 39(32.8%) had moderate malaria density (1000 – 9999/µl or ++), 3(2.5) had severe malaria density (>9999µ/l or +++), while 26(24.3%) had malaria parasites of different densities in addition to one or two other disease condition(s) like diabetes (3), HIV (5), hypertension (6) and typhoid (12). Controls were 40 apparently normal pregnant women of the same age range and within the same trimesters. These control subjects, who were on routine antenatal visits, had no observable clinical symptoms and, on screening, were negative for malaria parasites and other disease conditions mentioned earlier. All the subjects were singleton from their ultrasound results, while 76(63.9%) of the patients and 26(65%) of the controls were either primigravidae or secundigravidae.
2.3. Exclusion Criteria
- All patients without complaints relating to malaria infection were not considered for recruitment for the study as patients. Those with severe malaria density were also excluded from the study, because the number obtained (3) within the time of the study was not enough for statistical analysis. Also excluded were those with other disease conditions in addition to malaria infection, to make sure that changes in the levels of these antioxidant minerals were mainly due to malaria parasitemia.
2.4. Dietary Index
- The dietary indices of the subjects were assessed as earlier reported [21]. The results showed that they have similar dietary indices.
2.5. Laboratory Analysis
- After counseling, informed consent of each of the patients was obtained and 6.0ml of blood sample was collected through the ante-cubital vein. Out of this, 5.0ml was put into a chemically clean glass test tube while 1.0ml was put into sequestrene bottle. From the later, thick and thin blood films were made, allowed to air-dry and stained with Giemsa and leishmann stains respectively, while the remaining was used to determine total white blood cell count. Determination of absolute malaria parasites count was done by counting the number of parasites against 200 leucocytes in a well made thin film and then calculated as follows
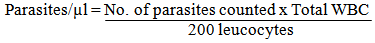 Thick films, which were observed under oil immersion lens, were used to confirm the parasite counts and also to rule out possible false negative results from the thin films. The results from the thick films were presented in notations of + to +++. They were related to the absolute parasite count as earlier reported [4]. The sample in plain test tube was allowed to clot and retract before centrifuging at 5,000 rpm for 10 minutes. The serum obtained was used to determine the concentrations of copper, manganese, selenium and zinc using atomic absorption spectrophotometer (Buck Scientific Spectrophotometer, Model 205, East Norwalk, Connecticut, USA).
Thick films, which were observed under oil immersion lens, were used to confirm the parasite counts and also to rule out possible false negative results from the thin films. The results from the thick films were presented in notations of + to +++. They were related to the absolute parasite count as earlier reported [4]. The sample in plain test tube was allowed to clot and retract before centrifuging at 5,000 rpm for 10 minutes. The serum obtained was used to determine the concentrations of copper, manganese, selenium and zinc using atomic absorption spectrophotometer (Buck Scientific Spectrophotometer, Model 205, East Norwalk, Connecticut, USA).2.6. Statistical Analysis
- Statistical analyses were done using Graph Pad Prism 5.0 series. Values were expressed as means and standard deviations. Differences between means were calculated using one-way analysis of variance (ANOVA) and significance was taken at p<0.05.
3. Results
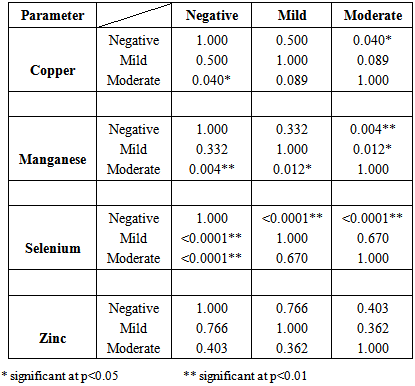
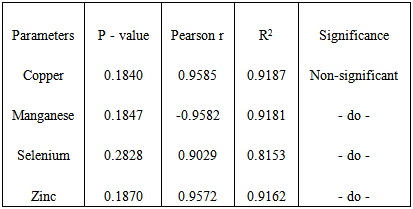
- Table 1 shows the means ± standard deviations of all the antioxidant minerals obtained in controls and patients (at two different malaria densities) – 1.36±0.78, 1.44±0.65 and 1.69±0.68 for copper; 0.60±0.53, 0.53±0.43 and 0.31± 0.26 for manganese; 78.93±16.63, 95.55±19.86 and 98.16±12.41 for selenium and 2.69±1.33, 2.74±0.81 and 2.90±0.76 for zinc. The table also shows the analysis of variance of the mean values. From the table, while copper and zinc showed relative but non-significant changes as malaria density increased, manganese and selenium showed significant changes. Table 2 is the result of multiple comparisons of all the parameters at different malaria densities. The table shows that changes in copper concentration were significant only between control and moderate density (p=0.040), manganese and selenium showed significant changes at different levels – either between the control and mild density, between the control and moderate density or between the mild and moderate densities, while zinc showed no significant changes at any level of comparison. Table 3 is the results of the correlation study between malaria density and each of the mineral antioxidants. Though all the correlations are relative and non-significant, copper and selenium showed positive relation while manganese and zinc showed negative relation.
|
|
|
4. Discussion
- Copper concentration in non-parasitized pregnant women (controls) obtained from this study is higher than the figures reported in healthy non-pregnant women from different parts of this country [19, 22]. The difference may be as a result of the effect of pregnancy on the trace element, since many studies have reported significant and non-significant increased copper concentration in pregnancy, especially in multiparous pregnant women [21-25]. However, the finding of relative but non-significant increase (P=0.0970) in copper concentration in parasitized pregnant women in this study falls between the results of some earlier studies that reported significant decrease [22, 26, 27], and significant increase [28] of the same antioxidant in malaria parasitemia. Copper is an integral component of many metallo-enzymes that play significant roles in antioxidant protection, including prevention of production and accumulation of free radicals, and scavenging and inactivation of already produced free radicals [29]. Three factors may play in favour of increased copper concentration in parasitized pregnant women. Firstly, copper and its carrier protein – caeruloplasmin, are acute phase reactants [30, 31] and may respond positively to the inflammatory activities of malaria parasites. Secondly, during pregnancy, estrogen induces the synthesis of caeruloplasmin which, in turn, increases the serum level of copper [32]. Thirdly, in pregnancy, serum copper increases two to three folds over normal concentration during the last trimester, especially in multiparous women [21, 23, 24]; hence, pregnant women hardly have copper deficiency even in the presence of other pathological conditions like gestosis, intrauterine growth retardation and preterm labour [24]. From the foregoing therefore, finding of significant or non-significant increase in copper concentration in parasitized pregnant women, which is a sign of oxidative stress, is more likely than the reverse. While the second and third factors may account for the difference in copper levels of pregnant and non-pregnant women as earlier reported [19], the first factor increases it further in parasitized pregnant women. This further increase is expected to be very beneficial to the patients in contending with free radicals generated by the parasites’ invasion.From this study, manganese decreased significantly (P=0110) in malaria-parasitized pregnant women. Presently, there is dearth of literature on the levels of manganese in malaria parasitemia. However, it is generally accepted that manganese concentration increases significantly during pregnancy and from one trimester to another, especially in non-supplemented and multiparous women [21, 33, 34], despite the oxidative stress caused by pregnancy. Manganese is a known activator that promotes enzyme functions in a multitude of ways. For instance, it is a component of many powerful antioxidant enzymes like glutathione synthetase and mitochondrial based manganese-dependent superoxide dismutase [23, 35-37]. Therefore, the result of this study – significant decrease (P=0.0110) in malaria infection during pregnancy, is likely due to the effect of malaria parasitemia, that is, increased utilization of the element to activate manganese-dependent antioxidant enzymes for fight against oxidative stress. This may have many medical implications. Firstly, since magnesium and cobolt can substitute for manganese in some enzyme systems, allowing some body functions in deficiency states [37], manganese deficiency will, at long term, lead to deficiencies of magnesium and cobolt, causing other medical problems. Thus, pregnant women with malaria may be prone to, other conditions like epileptic fit and exocrine pancreatic insufficiency due to magnesium deficiency and microcytic anaemia due to cobolt deficiency, while their new born babies may later develop inborn errors of metabolism like phenylketonuria and maple syrup disease, and abnormal brain development [38, 39]. Therefore, adequate concentration of manganese during malaria infection, through supplementation as treatment adjuvant or enhanced nutritional intake, will save magnesium and cobolt for their specific functions, prevent the development of other diseases and also allow for maximum functions of manganese-dependent enzymes. Secondly, though high exposure to manganese during pregnancy is suspected to have toxic effects on the developing fetus [40], non-linear relation has been found between maternal manganese and birth weight [41], suggesting that manganese deficiency may affect fetal growth. Thus, the decrease in manganese concentration in malaria parasitemia may cause multi-factorial problems, including abnormal fetal growth, while calculated supplementation can prevent many medical problems without obvious toxic effects.This study showed significant increase (P<0.0001) of selenium concentration in parasitemic pregnant women. Selenium is known to have many functions including anti-oxidation and immune functions, and its importance increases in all ages [42, 43]. It is an integral part of many antioxidant enzymes used by the body to fight inflammations. For instance, natural selenium-containing antioxidant enzymes – glutathione perioxidase and thioredoxin reductase, are known to protect neutrophils, macrophages and other tissues from free radicals intended to destroy pathogens in the fight against inflammation [44]. Hence, neutrophils with reduced glutathione peroxidase activity due to selenium deficiency were found to be unable to defend themselves against free radicals they release onto pathogens [45, 46]. Though selenium is known to progressively accumulate in cord blood throughout gestation, resulting to positive correlation between gestational age and cord blood concentration [47, 48], there is limited transfer of the element across placenta, making fetal concentration constantly low [43]. Thus, in selenium deficiency in pregnancy, low selenium concentration in fetal blood is caused by two major factors – maternal deficiency and limited transfer across placenta. This often leads to many pathophysiologies known to be major medical complications of preterm and very low birth weight infants [49]. Though selenium is a component of some antioxidant enzymes, it is also an antioxidant of its own, and it is said to decrease progressively throughout gestation [18]. Therefore, its increase in parasitemic pregnant women may be due to increased mobilization of the element to fight inflammation caused by parasites’ invasion. Furthermore, immune status and selenium concentration are said to increase as parity increases [16, 18], making primigravidae and secundigravidae more susceptible to malaria attack than multigravidae. Thus, increased selenium concentration in parasitemic pregnant women may be ascribed to increased response to inflammatory activities of malaria by the primigravidae and secundigravidae who made up the greater percentage of our study subjects (63.9% of patients and 65% of the controls). Therefore, the finding of increased selenium concentration in parasitemic pregnant women may be helpful in stemming down the pathophysiologies associated with malaria parasitemia in pregnancy. Moreover, recent study [50] also demonstrated the beneficial effects of selenium as an adjuvant in the treatment of malaria in children; hence we dare to say that increased selenium concentration in parasitemic pregnant women, which may be an inflammatory response, is beneficial to both the pregnant mother and the unborn child. In spite of the increase, we still advocate for simultaneous assessment of selenium status in malaria in pregnancy to avoid isolated low level and to ensure adequate supplementation if need be.Like selenium, serum zinc concentration increased, but non-significantly (P=0.6470), in parasitemic pregnant women. Though this result was not in agreement with the findings of some previous studies [28, 51] that reported significant decrease in parasitemia, these earlier studies were done on children. Therefore, the current result can be ascribed to inflammatory response leading to increased mobilization of the element, which may not have been well developed in infants used in the previous studies. Zinc plays very important and critical roles in various functions of the body including immune modulation (enhancing both innate and acquired immunity) and metabolism of other micronutrients [52, 53]. In immune function, it enhances lymphocyte function implicated in resistance to malaria and increases microbial activities of macrophages [54, 55]. Zinc is known to decrease significantly over gestation, causing its requirement in third trimester of pregnancy to be approximately twice that of non-pregnant women [18, 56]. This deficiency during pregnancy has been implicated in many adverse pregnancy outcomes, including delivery of small-for-age babies in areas known to have zinc deficiency [18, [57-59]. Because of its role in immune modulation, the significant increase in malaria parasitemia is an indication of response to the challenge of oxidative stress caused by malaria parasites. However, the increase was not progressive, and this may be as a result of two effects; one, continued utilization of the element for the fight against oxidative stress, and two, negative effect of pregnancy on the element.From the foregoing, we are of the opinion that symptomatic malaria parasitemia causes additional oxidative stress in pregnant women. However, this study would have been heavier if it was a longitudinal one, extending from the onset of the clinical signs of the infection to about one to two weeks of complete clearance of the parasites from the patient. Additional work also needs to include pregnant women with high density parasitemia, while subjects need to be categorized according to their gestational ages and parity. All these shall be taken into consideration in subsequent studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML