Ruthashini R Selvasingam
Faculty of Medicine, Universitas Udayana, Denpasar, Bali, Indonesia
Correspondence to: Ruthashini R Selvasingam, Faculty of Medicine, Universitas Udayana, Denpasar, Bali, Indonesia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Traveler's diarrhea is one of the most common gastrointestinal illnesses among the travelers all over the world. Diarrhea, caused by enteric infections contributes to the increase in morbidity and mortality worldwide. It is estimated around 2-4 billion episodes of infectious diarrhea occur each year. This review highlights on Enterotoxigenic Eschericha Coli (ETEC) as the main cause of traveler’s diarrhea. The underlying mechanism of bacterial diarrhea discussed includes the pathway in how ETEC causes diarrhea which is through intestinal colonization, followed by elaboration of diarrhegenic enterotoxin. The mainstay of treatment for traveler's diarrhea would be oral fluid in preventing dehydration. Medications such as antibiotic have shown to be effective. The primary control strategy for ETEC is preventing fecal-oral transmission.
Keywords:
Travellers diarrhea, ETEC, Mechanism of diarrhea, Management
Cite this paper: Ruthashini R Selvasingam, Enterotoxigenic Escherichia coli (ETEC) as the Cause of Traveler’s Diarrhea, American Journal of Medicine and Medical Sciences, Vol. 4 No. 5, 2014, pp. 154-160. doi: 10.5923/j.ajmms.20140405.03.
1. Introduction
Traveler’s diarrhea is one of the most common gastrointestinal illnesses among the travelers all over the world. Thirty percent to seventy percent of travelers may develop diarrhea depending on the region of the world they visit. Diarrhea is the most common illness among travelers which affects 10 million people each year, according to Centers for Disease Control (CDC). Traveler's whom are at risk for diarrhea usually come from industrialize nations and travel to high risk areas including Asia, Africa, the Middle East, Mexico, Central and South America. Areas of lesser risk include Eastern Europe, South Africa, China and some Caribbean nations. Travel to areas of the United States, Canada, Northern and Western Europe, New Zealand and Australia pose the lowest risk to travelers [1].Traveler’s diarrhea is commonly present with symptoms like nausea, abdominal cramps, and bloating. Travelers' from temperate climate of the world usually experience diarrhea between four days to two weeks after arriving. Based on epidemiologic data, both men and woman are at equal risk for developing travelers' diarrhea. Younger individuals are more commonly afflicted, perhaps because of more adventurous eating habits [2]. Traveler’s diarrhea are normally due to consuming food contaminated with bacteria or, less commonly, with parasites or viruses. The largest contributor to the risk for traveler’s diarrhea is likely due to poor hygiene practiced in local restaurants.
2. Literature Review
2.1. Etiology of Traveler’s Diarrhea
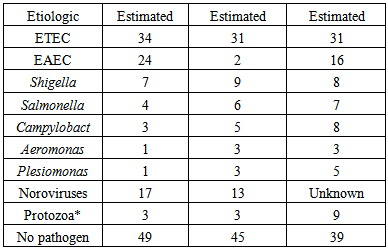
Traveler’s diarrhea is currently the most common illness among travelers all around the world (Figure 1) [3]. There are many causes of diarrhea, including bacterial, viral, and parasitic pathogens (Table 1). The main cause of traveler’s diarrhea is bacteria. The common bacteria causing this problem are Escherichia coli, Shigella, Camplyobacter, Salmonella, Aeromonas, Plesiomonas and noncholera Vibrios [2]. ETEC can be identified in around 80% of cases worldwide contributing to the most number of cases [4].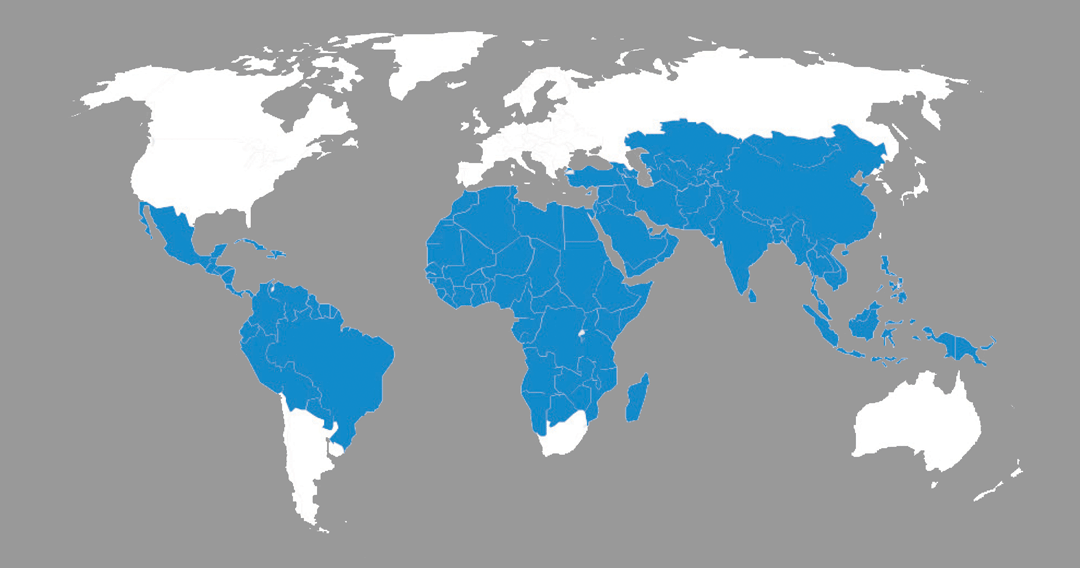 | Figure 1. Countries at high risk for traveler’s diarrhea (WHO, 2008) |
Table 1. Causative Agent in Traveler’s Diarrhea (Bauche & DuPont, 2011)
 |
| |
|
ETEC and Enteroaggregative E.coli (EAEC) are the main contributors for cases occurring in Latin America, Africa, South Asia and Middle East (Figure 2). In South and Southeast Asia, Shigella, Camplyobacter and, Salmonella are very important contributors for traveler’s diarrhea cases. Travelers to Africa and Latin America are affected by noroviruses. Aeromonas and Plesiomonas contributes 10% to the number of traveler’s diarrhea cases in all high risk countries. Arcobacterbutzleri causes 8% of traveler’s diarrhea cases in Mexico, Guatemala, and India. Consumption of fruits and vegetables high in Cyclosporacayetanensis causes diarrhea in international travelers [2].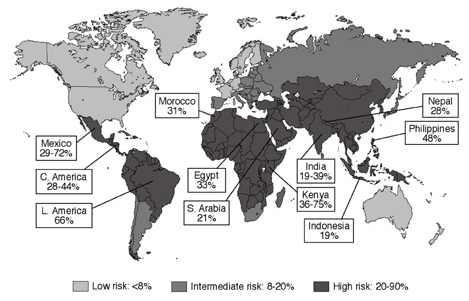 | Figure 2. Percentage incidence of travelers' diarrhea caused by Enterotoxigenic Eschericia coli (WHO, 2008) |
Norovirus, rotavirus and astrovirus are the main viral pathogens which contribute to viral diarrhea. A very rare cause of diarrhea is parasitic infections with the exception of Giardia lamblia. Cryptosporidum, and another parasite, which has been implicated as a common cause of diarrhea. Cyclospora is highly dependent on geographical and seasonal variability. Dientamoebafragilis is a persistent pathogen that occasionally diagnosed in travelers [1].
2.2. Enterotoxigenic E. coli (ETEC) as the Main Cause of Traveler’s Diarrhea
Travelers' diarrhea usually is caused by the consumption of polluted food or water. Food is considered to be the main cause of traveler's diarrhea if compared to water. The CDC estimates up to 80% of cases of travelers' diarrhea are caused by bacteria [3]. Enterotoxigenic E., one of five classes of enterovirulent E. coli, is the most common bacterium that causes travelers' diarrhea [5].E. coli is a gram negative, rod shaped bacterium with numerous serotypes, most of which normally inhabit the human intestinal tract with little ill effect. Several strains, however, produce toxins that work on the intestinal lining and causes disease. These five classes of enterovirulent E. coli are known as the EEC group (enterovirulent E. coli). Each class of EEC is distinguishable and different from the others. There are five major classes of diarrheogenic E coli: enterotoxigenic (ETEC), enteropathogenic (EPEC), enterohemorrhagic (EHEC), enteroaggregative (EAEC), and enteroinvasive (EIEC) (Table 2) [5].Table 2. Escherichia coli in diarrheal disease (Bloch, 2006)
 |
| |
|
EnterotoxigenicE.coli (ETEC) is the major contributor of traveler’s diarrhea worldwide. Transmission of ETEC occurs when a person drinks water or eats food contaminated with ETEC bacteria. The main source of ETEC contamination are human or animal waste such as feces. Its estimated importance in Latin America is 34%, in Africa is 31% and South Asia (Indian Subcontinent) is 31% [2]. In one study conducted among United State travelers returned from Mexico, it was identified that 19% to 40% of these traveler’s stool specimen contain ETEC. ETEC identified to produce two types of enterotoxins which are heat-labile toxin (LT) and heat stable toxin (ST) or both at the same time (LT/ST). Strains isolated from travelers who visited Mexico produce LT alone or in combination with ST approximately 34% to 68% [6].
2.3. Pathogenesis of ETEC Colonizing Mucosal Surface of Small Intestine
Diarrheal disease caused by ETEC is marked by a rapid onset of non-bloody, watery diarrhea, accompanied by little or no fever (Figure 3). Abdominal pain, malaise, nausea, and vomiting are the other common symptoms. Diarrhea and other symptoms stop spontaneously after 24 to 72 hours [7]. | Figure 3. Pathogenesis of E coli diarrheal disease (Evans & Evans 1996) |
Transmission of ETEC bacteria is via the fecal-oral route. Pili (fimbriae) allow the bacteria to invade the ileal mucosa. Cytotonic enterotoxins (encoded on plasmid or bacteriophage DNA) initiate watery diarrhea. Plasmid- encoded invasion factors allow invasion of the mucosa, and plasmid- or bacteriophage-encoded cytotoxic enterotoxins initiate tissue damage. When either one of these factors emerge, it stimulate a host inflammatory reaction with an influx of lymphocytes and thus resulting in dysentery [5].Pathogenesis of ETEC bacteria invasion involves two steps which are intestinal colonization, followed by elaboration of diarrheagenic enterotoxins (Figure 4) [7]. First ETEC must colonize the small intestine to cause disease. Then, the pilli liaise attaches to specific receptors on villous enterocytes. The bacteria are able to multiply and form micro colonies once attached to specific receptors. When the colonization is complete, the bacteria produces toxins which causes the diarrhea [8].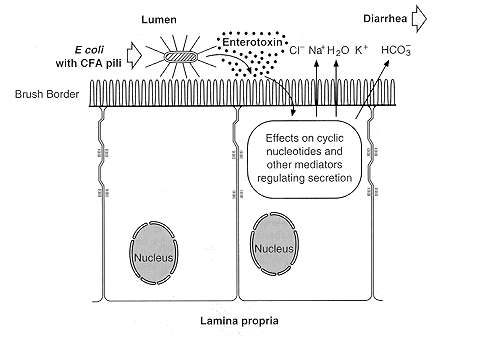 | Figure 4. Cellular pathogenesis of ETEC having CFA pili (Evans & Evans, 1996) |
ETEC produces two types of enterotoxins which are heat-labile toxin (LT) and heat stable toxin (ST) [6]. Heat stable toxin (ST) is actually a group of toxic peptides containing 18 to 50 amino acid residues in length. The STa toxin can induce intestinal guanylatecyclase, the enzyme that converts guanosine 5′-triphosphate (GTP) to cyclic guanosine 5′-monophosphate (cGMP). Increased intracellular cGMP restrict intestinal fluid uptake, resulting in net fluid secretion. Those termed STb do not seem to have the same effect in causing diarrhea. One method for testing suspect E. coli isolates for ST production involves injection of culture supernatant fluids into the stomach of infant mice and seeing whether diarrhea occurs. Specific DNA gene probes and PCR assays have been developed to test isolated colonies for the presence of genes encoding ST and LT [5, 7].LT can be divided into two serogroups which are LT I and LT II where both of these do not cross react immunologically. LT I can be seen in strains of E.coli that are pathogenic for both animals and humans. On the other hand, LT II is rarely found in human isolates and has not been associated with disease. LT1 and LTII mimics CT structurally and functionally. LT1 made of enzymatic Asubunit as well as pentameric B subunit. Both of these subunit attached strongly to ganglioside GM1.GM1 (monosialotetrahexosylganglioside) ganglioside binds B subunit to the surface of cell of mammalian and allows introduction of A subunit to the host cell (Figure 5). Cascade of events are initiated by subunit A resulting in watery diarrhea. However, B subunit binds to GM1 ganglioside and a different set of events take place. Adenylatecyclase is being the target of LT, causes increase in cAMP, activates PKA and leads to phosphorylation and activation of CFTR. The increase stimulation in Cl-secretion is an example of how LT and CT cause diarrhea. LT causes decrease in H+/PEPT co-transporter through a cAMP dependent pathway in Caco-2 cells. Thus, hypersecretory effects of LT is mediated by cAMP resulting in decreased absorption and increased secretion of fluids and electrolytes [5, 6].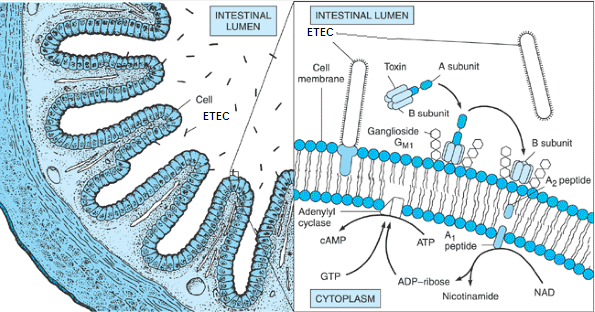 | Figure 5. Pathogenesis of enterotoxigenicE. coli (ETEC) in diarrheal disease. The bacteria gain entry to the small intestinal lumen through ingestion of contaminated food (left). They elaborate an enterotoxin that is composed of one A subunit and five B subunits. The B subunits bind to the intestinal cell membrane and facilitate entry of part of the A subunit (right). Subsequently, this results in a prolonged activation of adenylyl cyclase and the formation of cyclic adenosine monophosphate (cAMP), which stimulates water and electrolyte secretion by intestinal endothelial cells. (Bloch, 2006) |
Lipopolysaccharide and gamma interferon-induced tumor necrosis factor alpha and IL-12 are suppressed by LT. IL-10 secretion by macrophages are enhanced. LT modulate immune response in part by enhancing production of IL-10. This explains why travelers with genetically predisposed of producing IL-10 are more prone to suffer from diarrhea when exposed to ETEC LT [6].
2.4. Management
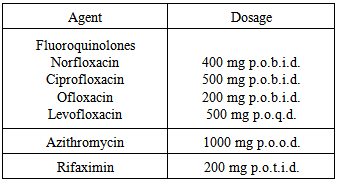
Three antibiotics have clearly demonstrated effectiveness in reducing duration and severity of traveler’s diarrhea. These drugs are fluoroquinolone (ciprofloxacin or levofloxaxin), rifamixin and azithromycin (Table 3) [9, 10]. Table 3. Recommended antibiotics for the treatment of traveler’s Diarrhea (Saussure, 2009)
 |
| |
|
Fluoroquinolone is important in empirical therapy and remain drug of choice for prophylaxis and treatment of bacterial traveler’s diarrhea pathogen but increasing resistance may hinder its use in future [1]. Its level of resistance develops over time, reaching 58% for the 1998-2003 periods. Its not recommended in pregnant and lactating women [9]. Azithromycin, a macrolide is recommended for self-treatment among traveler’s suffering from diarrhea because it is affective against most of the bacterial pathogens [9, 10]. Azithromycin has a high degree of activity against diarrhea due to ETEC and EAEC [10]. Its safe to be used in children and pregnant women [9].Rifamixin derived from rifampin has been approved for treatment of traveler’s diarrhea caused by noninvasive E. coli in travelers more than 12 years old. Rifamixin is non-absorbed, and has the same efficacy as absorbed ciprofloxacin. It produces less unwanted effects [10]. Alternatively, anti-motility agents such as loperamide (Imodium) offers the most impressive antidiarrheal effects by reducing the number of stools passed around 60%. Added clinical benefit obtained when loperamide combined with anti bacterial treatment. The dosage recommended is 4 mg (in 2 capsules) initially followed by 2 mg after each unformed stool but not to exceed 8 mg/day [11].Oral fluids are very important as they play a role in preventing dehydration. Oral rehydration salts (ORS) such as World Health Organization ORS can be used for severe fluid loss. ORS is prepared by adding I packet to the appropriate volume of boiled or treated water. If the patient unable to drink enough fluids to prevent dehydration, he/she may need to receive fluids intravenously [1]. Figure 6 outlines recommended treatment strategies based upon clinical symptoms and severity of problem. These treatment strategies are formulated according to three stages of traveler’s diarrhea which are mild, moderate and severe. Travelers with mild illness do not have to alter his or her schedule of travelling. Travelers with moderate illness have to make amendment to their travel schedule but they are not disabled. Travelers with severe illness are disabled. They have fever or are passing bloody stools, or are passing unformed stools more than 6 times per day. If the patient has severe illness but has no fever more than 101°F or does not pass bloody stools, they can be treated using moderate illness treatment strategy [2]. 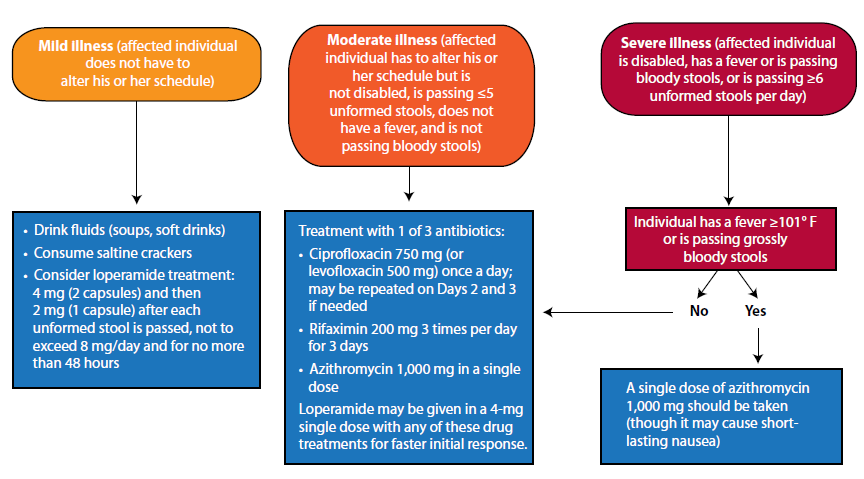 | Figure 6. Treatment algorithm for the management of traveler’s diarrhea (Bauche & DuPont, 2011) |
2.5. Prevention
The primary control strategy for ETEC is by preventing oral-fecal transmission through building sanitation infrastructure. Variety of measures can be taken to control infection, these include cooking food and keeping it hot, peeling fruits and vegetables, and using water that has been boiled or chemically treated with iodine, chlorine, or another disinfectant [1].Rifaximin, a derivative of rifamycin which is essentially nonabsorbed, awaits its FDA approval to be used in the United States, have a role in prophylaxis. Rifaximin chemoprophylaxis may be given to prevent illness during travel to areas with high risk and is especially effective in combating diarrhea due to ETEC. Azithromycin could be added to this prophylaxis for better outcome [10]. Rifaximin reduced the risk for traveler’s diarrhea in travelers to Mexico by 58-77% [2]. Probiotics help to improve the GI microbial balance as they are live microbial food supplements that beneficially affect the host animal. It has been tested as preventive agents against traveler’s diarrhea. Lactobacillus GG (LGG) and Saccharomyces boulardii has been distinguished by its resistance to sterilization by gastric acid and bile and its ability to adhere to and colonize bowel, has been found to be more promising as a prophylactic agent for traveler’s diarrhea [2]. Bismuth subsalicylate (BSS) preparations (essentially an antidiarrheal agent) have been effective is reducing ETEC and other common bacterial infections that causes diarrhea. Bismuth subsalicylate, may be effective as a prophylactic agent as it has shown to prevent 60% to 65% of cases of traveler’s diarrhea. Bismuth are required to be taken 4 times daily in acquiring a full preventive effect. Bismuth can be associated with several side effects such as tinnitus and blackening of the tongue and stool [11].None of the vaccines directed against ETEC bacteria are on market at this period. Dukoral is an oral whole-cell/recombinant B-subunit vaccine which is directed against cholera. Dukoral has found to be effective in providing short term efficacy against ETEC diarrhea. Dukoral's protective efficacy against cholera is 85 percent. Nevertheless, its protection against the heat-labile toxin of ETEC reaches 67 percent [2]. Heat-labile toxin (LT) skin patch application of ETEC is a new development in vaccine against ETEC. Application of the patch plus LT by abrasion of stratum corneum produces a brisk serum antitoxin antibody response. When a phase II trial was done, it was seen that LT patch provided greater-than-70% protection against moderate and severe traveler’s diarrhea cases conducted in Mexico and Guatemala. The LT Patch is now being evaluated in a phase III study of immunized subject travelleing from Europe to Mexico and Guatemala. An oral attenuated ETEC vaccine strain is in development. This strain has shown to be immunogenic [2].
3. Conclusions
Traveler’s diarrhea is currently the most common illness among travelers all around the world. The main cause of traveler’s diarrhea is bacteria. The common bacteria causing this problem are Escherichia coli, Shigella, Camplyobacter, Salmonella, Aeromonas, Plesiomonas and noncholera Vibrios. Norovirus, rotavirus and astrovirus are the main viral pathogens which contribute to viral diarrhea. A very uncommon cause of traveler's diarrhea is parasitic infections with the exception of Giardia lamblia and Cryptosporidum. The main bacterium (80%) that causes travelers' diarrhea is enterotoxigenic E. coli, one of five classes of enterovirulent E. coli. ETEC can be transmitted through food, or drinking water or ice which are contaminated with ETEC bacteria. NoVs were the second most (10.2%) common enteropathogen identified in travelers following diarrheagenic E. coli.There are two steps involved in the pathogenesis of ETEC: intestinal colonization, followed by elaboration of diarrheagenic enterotoxins. The bacteria gain entry to the small intestinal lumen through ingestion of contaminated food. ETEC identified to produce two types of enterotoxins which are heat-labile toxin (LT) and heat stable toxin (ST). The heat-labile toxin (LT) activates adenylyl cyclase in a manner analogous to cholera toxin.The heat-stable toxin (ST) activates guanylylcyclaseactivity. They elaborate an enterotoxin that is composed of one A subunit and five B subunits. The B subunits bind to the intestinal cell membrane and facilitate entry of part of the A subunit. There is a relationship between symptomatic ETEC LT and Interleukin 10 (IL-10) alleles associated with high IL-10 infection.Antibiotics are used to treat traveler’s diarrhea caused by ETEC. Three antibiotics which are fluoroquinolone, rifamixin and azithromycin have clearly demonstrated effectiveness in reducing duration and severity of traveler’s diarrhea. Alternatively, anti-motility agents such as loperamide (Imodium) offers antidiarrheal effects by reducing the number of stools passed around 60%. Oral fluids are one the main therapy since they are important in the prevention of dehydration. Prevention of fecal-oral transmission is the primary control strategy which is done through building sanitation infrastructure. Rifamycin, probiotics and Bismuth subsalicylate (BSS) can be used to prevent diarrhea caused by ETEC. Currently there is no vaccine available for ETEC. Skin patch LT are shown to effective in combating against ETEC.
ACKNOWLEDGEMENTS
First of all, I would like to show my gratitude to the Lord because with his blessings I have managed to complete my paper entitled "Enterotoxigenic Escherichia Coli (ETEC) as the causes of Traveler’s Diarrhea".I take this opportunity to express my profound gratitude and deep regards to my guide to dr. Ida AyuIka Wahyuniari, M. Kes, for her exemplary guidance, monitoring and constant encouragement throughout the course of this thesis. The blessing, help and guidance given by her time to time shall carry me a long way in the journey of life on which I am about to embark. I am obliged to staff members of Udayana University, for their valuable information provided by them in their respective fields. I am grateful for their cooperation during the period of my assignment.Lastly, I hope that my writing would be beneficial to the readers as how it was for me.
References
| [1] | Connor BA. Travelers’ Diarrhea, Chapter 2: The Pre-Travel Consultation Self-Treatable Conditions, 2012 Yellow Book, Traveler’s Health, Centers for Disease Control and Prevention. 2012:39-48. |
| [2] | Bauche JDLC and DuPont HL. New Developments in Traveler’s Diarrhea. Gastroenterology & Hepatology. 2011; 7(2):88-95. |
| [3] | WHO. The World Health Report 2008: Primary Health Care-Now More Than Ever. 2008. |
| [4] | Goldsmid JM and Leggat PA. The Returned Traveler with Diarrhea. Australian Family Physician. 2007;36(5):322-327. |
| [5] | Bloch KC. Infectious Diarrhea, Chapter 4 Infectious Diseases, Pathophysiology of Disease, Lange, 5th edition. McGraw-Hill Medical, United States of America. 2006:80-83. |
| [6] | Flores J, DuPont HL, Lee SA, Jaime BG, Paredes M, Mohamed JA, Armitige LA, Guo DC and Okhuysen PC. Influence of Host Interleukin-10 Polymorphisms on Development of Traveler’s Diarrhea Due to Heat-Labile Enterotoxin-Producing Escherichia coli in Travelers from the United States Who Are Visiting Mexico. Clinical and Vaccine Immunology. 2008;15 (8):1194–1198. |
| [7] | Evans DJ and Evans DG. Noninflammatory Diarrheas Caused by Enterotoxigenic Escherichia Coli, Chapter 25 Escherichia Coli in Diarrheal Disease, Medical Microbiology, 4th edition. University of Texas Medical Branch, United States of America. 1996: 245-254. |
| [8] | Huang DB &Nataro JP. Norovirus (Norwalk and Norwalk like Virus), Chapter 6 Pathogenesis, Traveler’s Diarrhea, 2nd Edition. People’s Medical Publishing House, United States of America. 2008;2:64-66. |
| [9] | Saussure PPHD. Management of the returning traveler with diarrhea. Therapeutic Advances in Gastroenterology. 2009;2(6):367-375. |
| [10] | DuPont HL. Azithromycin for the Self-Treatment of Traveler’s Diarrhea. Clinical Infectious Diseases. 2007; 44:347–349. |
| [11] | DuPont HL. Therapy for and Prevention of Traveler’s Diarrhea. Clinical Infectious Diseases.2007;45:S78–84. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


