-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2014; 4(3): 96-101
doi:10.5923/j.ajmms.20140403.03
Do Marital Habits Affect Epilepsy Classifications and Etiologies? Update from Epilepsy Registry
1College of Medicine, Department of internal medicine, King Khalid University, Abha, Saudi Arabia
2Division of Neurology, Department of Internal medicine, Aseer Central Hospital, Abha, Saudi Arabia
Correspondence to: Fawzi A. Babtain, College of Medicine, Department of internal medicine, King Khalid University, Abha, Saudi Arabia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
In this retrospective study, we evaluated 740 patients with epilepsy in Aseer Central Hospital, Abha, Saudi Arabia between January and December 2012, with the aim of identifying any effect exerted by the presence of parental consanguinity (PC) on epilepsy classifications and etiologies. PC was documented in 183 patients (24%). The presence of family history of epilepsy (FHE) was significantly recorded in patients with PC (38% vs 22%; p <0.0001). According to epilepsy etiology, idiopathic epilepsy was documented more in patients with PC (34% vs 27%; p = 0.056), while cryptogenic and symptomatic epilepsies were seen more in patients lacking PC (p > 0.05). MRI and EEG findings were not affected by the presence of PC. Therefore, PC has no apparent influence on epilepsy classifications, etiologies, or investigations, but was only associated with FHE. Lacking an influence of PC could be explained by the heterogeneous etiologies leading to epilepsy. Counseling families with epileptic members should not go beyond the usual counseling for FHE.
Keywords: Consanguinity, Family history of epilepsy, Idiopathic epilepsy, Epidemiology
Cite this paper: Fawzi A. Babtain, Do Marital Habits Affect Epilepsy Classifications and Etiologies? Update from Epilepsy Registry, American Journal of Medicine and Medical Sciences, Vol. 4 No. 3, 2014, pp. 96-101. doi: 10.5923/j.ajmms.20140403.03.
Article Outline
1. Introduction
- Consanguinity is a common marital habit practiced in many developing countries with variable rates among different tribes, ethnic groups and communities. It is defined as unions contracted between persons biologically related as second cousins or closer [1]. It is a rare practice in European countries [2] and considered a high risk marriage and subsequently illegal in the US since the 19th century [3, 4]. In Saudi Arabia, reports showed high rates of consanguineous marriage across regions [5], which was associated with mental retardation, neural tube defects and other hereditary neurological diseases [6-8]. Epilepsy, on the other hand, is a complex disorder characterized by a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain [9]. Etiologies of epilepsy are heterogeneous, ranging from acquired to hereditary factors, and are usually affected by the age when epilepsy started. Whether parental consanguinity (PC) is a risk for epilepsy remains in doubts, and its influence on epilepsy characteristics is even less clear [10, 11], for which we aimed to evaluate whether the presence of parental consanguinity (PC) exerts any effect on epilepsy classifications, semiologies or investigations.
2. Methods
- This is a retrospective analysis of 740 patients with epilepsy evaluated in Aseer Central Hospital, Abha, Saudi Arabia, conducted between January and December of 2012. Details of the hospital’s epilepsy registry and methods used to obtain the information had been described previously [12]. Aseer Central Hospital is the regional hospital serving an area of around 1.9 million people. Data were collected using the ongoing epilepsy registry in the hospital. Patients included in this study a) were 13 years and older; b) had two or more unprovoked seizures; c) had at least one surface electro-encephalogram (EEG); d) had magnetic resonance imaging (MRI) of the brain of 1.5T; e) and should have data on the presence or absence of parental consanguinity available at the time of the evaluation. Age at epilepsy onset was defined as the age of the first documented seizure, and was determined at presentation. All patients had routine EEG recording and brain MRI. EEG interpretation was provided by an epileptologist, and a radiologist provided reports on the MRI of the brain. Along with the clinical data, EEG and brain MRI were used to classify epilepsy and identify its etiology. The International Classification of Seizures and Epilepsies (ICES) was used to classified epilepsy according to etiology (idiopathic, cryptogenic and symptomatic) [14]. Idiopathic epilepsy was defined as epilepsy with no underlying structural brain lesion or other neurologic signs or symptoms, and presumed to be genetic. Cryptogenic (or probably symptomatic) epilepsy was defined as a syndrome believed to be symptomatic but with no identified etiology. Symptomatic (structural or metabolic) epilepsy consisted of epileptic seizures that resulted from 1 or more identifiable structural lesions of the brain [14, 15]. Reported epilepsy risk factors were identified in our patients [16]. Inquiries about the presence of PC were done for all patients, and in order to validate this information, the patient and his/her companion were both asked about the presence or absence of PC to ensure the accuracy of the information given. Consanguinity was identified as first and second degree consanguinity. At the same time, information about the presence of family history of epilepsy (FHE) was obtained, where patients were asked about the presence of epilepsy in a first or a second degree relative, which were defined according to the National Human Genome Research Institute [17]. FHE was considered positive if the family member was diagnosed with epilepsy, with a current or remote use of anti-epileptics. Patients were divided into two groups according to the presence or absence of PC, aiming to identify any influence of PC on epilepsy classifications and etiology. Statistical analysis was performed using IBM SPSS Statistics for Macintosh, Version 20.0.0, IBM Corp., Armonk, NY. Contingency tables and Fisher exact were utilized to calculate X2 or categorical data, and Student’s t-test was used for continuous variables, and confidence interval was obtained. P value was calculated, but because of multiple variables used, Bonferroni adjusted p value of <0.004 as a significant value.
3. Results
- Among the 740 patients, 385 men (52%) and 355 women (48%) were recorded. The mean age of epilepsy onset was 19 years (range; 1 month - 95 years, SD = 15 years). According to epilepsy etiology, idiopathic epilepsy was identified in 213 patients (29%), cryptogenic epilepsy in 249 patients (34%), and symptomatic epilepsy in 278 patients (38%). Seizure semiology was determined for the studied patients as follow; 54% (399 patients) had focal epilepsy, 34% (249 patients) had generalized epilepsy. In 12% of the cases (92 patients), seizure onset could not be determined for which their epilepsy was thought to be unclassified. Abnormal MRI of the brain was observed in 210 patients (29%). Epileptic discharges document in EEG was seen in 66% of patients (499 cases), where 197 patients (27%) had generalized discharges, 172 patients (23%) had focal discharges, and 119 patients (16%) had multifocal epileptic discharges in EEG. Epilepsy risk factors were determined (Figure 1). More than half of the patients had no identified risk factors. The presence of FHE was the most frequent risk factor identified, seen in one fourth of our studied population, a similar results to the previous report [12]. Some of the patients had more than one risk factor.
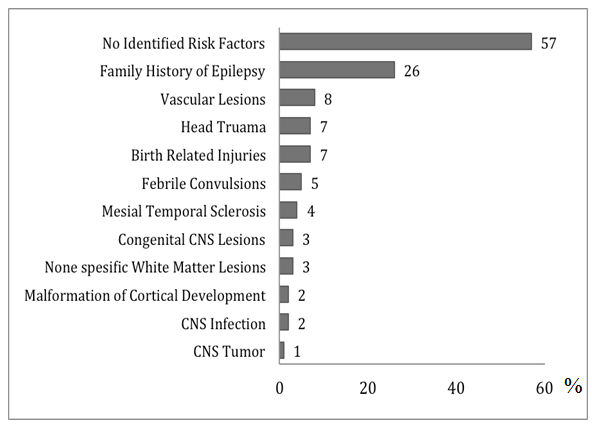 | Figure 1. Identified epilepsy risk factors (N= 740) |
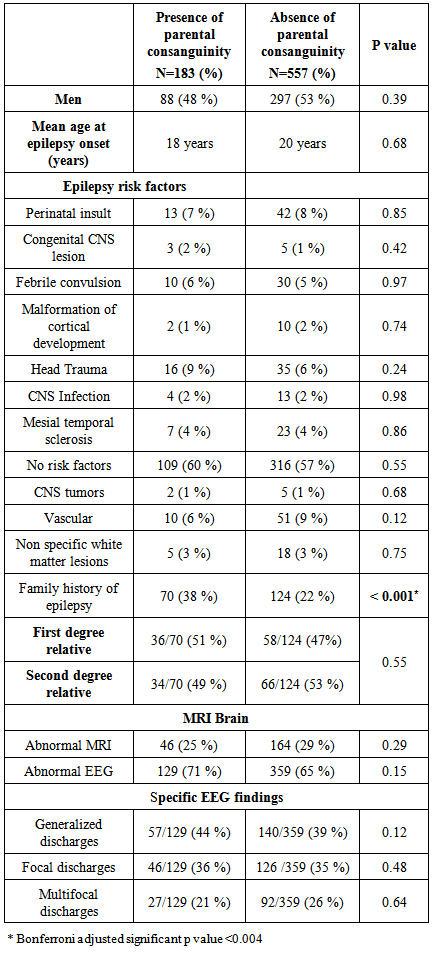
3.1. Consanguinity and Epilepsy Risk Factors
- Parental consanguinity of both degrees was documented in 24% of our patients (183 cases), where 13% (97 patients) had first degree PC and 12% (86 patients) had parents with second degree PC. Other forms of consanguinity were not analyzed in this study. Patients were further divided into two groups according to the presence or absence of PC (of either degree). Table 1 shows patient’s demographics and risk factors across the two groups. The age of epilepsy onset was slightly, but insignificantly, younger in patients with PC (18 vs 20 years, p = 0.096). Epilepsy risk factors were distributed between the two groups in no particular patterns, with the exception of the FHE, which was significantly seen in epileptic patients with history of PC (38% vs 22%, p <0.0001). Furthermore, the level of relationship of the epileptic family member in patients with PC was determined, and was compared to those with no PC. There was no association identified between the degree of relationship and the presence or absence of PC or its degrees (first or second degree PC) (Figure 2).
|
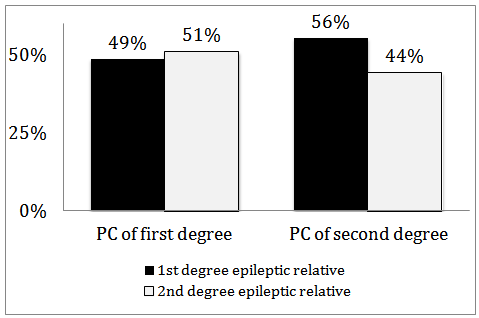 | Figure 2. Distributions of epileptic relatives (first or second degree) and degree of parental consanguinity (PC) |
3.2. Consanguinity and Epilepsy Etiology and Semiology
- Idiopathic (or genetic) epilepsy was seen in 34% of patients with PC and in 27% of those with no PC. This showed a trend for PC to be associated with idiopathic epilepsy, although this was not a statistically significant difference between the groups (p =0.056). Cryptogenic and symptomatic epilepsies were seen slightly more in patients lacking PC (Figure 2 A). Despite the insignificant abundance of idiopathic epilepsy in patients with PC, there was no significant impact of PC on the etiology of epilepsy. According to seizure semiology, partial epilepsy was the most frequent epilepsy type seen in both groups, yet slightly more in patients lacking PC (55% vs 51%, p = 0.35). Generalized epilepsy (primary or secondary) was observed 8% more in patients with PC (p = 0.07). Finally, unclassified epilepsy was the least frequent epilepsy type seen across the groups, and was documented more in patients with no history of PC (14% vs 9%, p =0.16). (Figure 2 B). The aforementioned observations revealed no significant effect exerted by PC on epilepsy semiology.
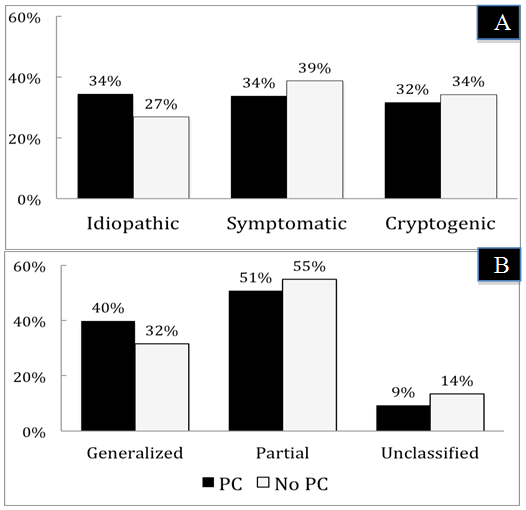 | Figure 3. Distributions of epilepsy etiology (A) and semiology (B) according to the presence or absence of parental consanguinity |
3.3. Consanguinity, MRI and EEG findings
- Epileptic EEG discharges were seen 6% more in patients with PC (71 vs 65%, p = 0.15). The distribution of specific EEG patterns was seen in both groups in no particular significance (Table 1). Therefore, consanguineous marriage didn’t affect the EEG patterns in patients with epilepsy. MRI of the brain was normal in more than two thirds of the patients in each group, slightly but insignificantly more in patients with history of PC (75 vs 71%, p = 0.3). One would conclude the absence of any influence of PC on the MRI findings of patients with epilepsy.
3.4. Predictors of Parental Consanguinity in Patients with Epilepsy
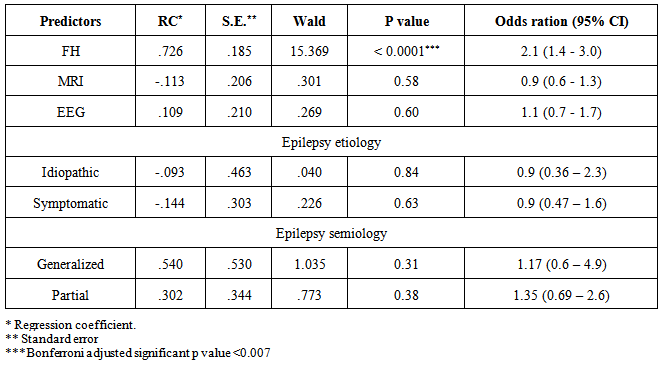
- Multivariate (binary) regression analysis was performed to predict the presence of parental consanguinity in epileptic patients. Factors used for prediction of consanguinity were epilepsy semiology (generalized, partial and unclassified) and etiology (idiopathic, symptomatic and cryptogenic), EEG findings, brain MRI findings, and the presence of FHE.Table 2 summarizes the results of the regression. Unclassified and cryptogenic epilepsies were excluded from the regression model by SPSS. Bonferroni adjusted p value of < 0.0071 was considered significant. Epileptic patients who had FHE were two times more likely to have PC of either degree (odds ratio = 2, 95% CI = 1.4-3.0, p < 0.0001). This result confirmed not only the association between FHE and PC but also the significant predictive role of the presence of FHE on PC among epileptic patients. Other factors failed to predict PC in patients with epilepsy.
|
4. Discussions
- Parental consanguinity across Arab countries ranged between 40-55%, and first degree PC was the most commonly practiced form of consanguinity [5, 18-21]. A community based study showed a strong association between PC and congenital heart disease (CHD) and other congenital malformations in children [22]. On the other hand, the prevalence of epilepsy in Saudi Arabia was comparable to other communities that had a lower incidence of PC, yet idiopathic and cryptogenic epilepsies contributed to 68% of the cases [23]. In another systemic review of epilepsy among the Arab countries, idiopathic epilepsy was represented in up to 82% of cases with epilepsy [24]. The aforementioned reports indicated higher rates of idiopathic generalized epilepsy in Arab nations compared to other societies, which might be related to marital habits but also other hereditary or environmental etiologies leading to epilepsy [25-28]. The present study showed that one fourth of the studied population had PC, which is similar to the reported literature, and its presence didn’t affect the age at epilepsy onset, unlike the influence observed by the presence of FHE from the same population [12]. There could be a small effect exerted by PC only on idiopathic epilepsy, but not other epilepsy etiologies, which might be related to the genetic effect of consanguinity on genetic epilepsy. The heterogeneous etiologies of epilepsy, particularly in the adult population, would dilute any genetic influence of PC on epilepsy for which a clear association between PC and genetic epilepsy could not be confirmed. There was no effect on epilepsy semiology, EEG patterns or brain MRI findings exerted by PC, and no gender difference was observed in patients with/without PC. Although the presented data were negative, it is the first to assess the role of PC in adult patients with epilepsy.A recent study by Babtain et al [12] analyzed the impact of family history of epilepsy (FHE) on epilepsy classifications and found that idiopathic epilepsy was strongly associated with the presence of FHE in patients with epilepsy, which may suggest a genetic influence exerted by FHE on epilepsy classifications. Generalized epilepsy (primary or secondary) was also significantly seen more in patients who had an epileptic family member, which may be attributed again to the genetic influence of FHE, as suggested by the authors. On the other hand, epileptic discharges documented in EEG were more likely to be seen in patients with FHE. The former findings indicated a significant impact of FHE on epilepsy classifications, semiology and EEG findings, which were not observed when analyzing PC and epilepsy. FHE was also associated with a younger age at epilepsy onset, and along with the other findings, this would strongly associated FHE with particular epilepsy types that started at younger age, mostly of an idiopathic generalized patterns, which were significant findings to indicate the effect of FHE but not PC.Family history of epilepsy is known to be associated with genetic epilepsy, and was present in higher rates across families with idiopathic epilepsy [29]. FHE was also found to be associated with idiopathic epilepsy and generalized EEG discharges [12], and it influenced the number of affected family members with epilepsy [30]. FHE is a known epilepsy risk factor in the adult and pediatric populations [31, 32], but data on the role of PC as a risk for epilepsy was conflicting [11, 33]. Idiopathic and cryptogenic epilepsies were associated with FHE more than PC in children with epilepsy [34, 35], and the presence of PC was not a predictor for epilepsy in these patients with cerebral palsy [36]. Our study showed a significant association between FHE and PC in epileptic patients, which could indicate clustering of epilepsy in families with PC, yet, the degree of relationship of the epileptic family member was not associated with PC or its degree. These results could not associate PC with a particular pattern of inheritance that would indicate a straightforward genetic role played by consanguinity on epilepsy etiology and pathogenesis. To our knowledge, this study is the first to evaluate the relation between PC and FHE across the adult onset epilepsy. Considering the negative effect of PC on epilepsy, one would conclude that counseling of epileptic patients or their parents should emphasize on the role on FHE as a risk for epilepsy and its association with genetic epilepsy.The present investigation has few limitations. We counted on self disclosure of PC by patients and their relatives, which could jeopardize the accuracy of obtaining such an information, although such a method was found to be reasonable in pervious reports [37]. Our epilepsy registry does not include patients younger than 13 years of age, who are evaluated separately by our pediatric neurology department. Including these young patients may alter some of these observations because of the higher incidence of genetic related epilepsy in the younger population. Finally, the prevalence of consanguinity in our study could be underestimated because individuals living in remote regions or in primitive cultures could face difficulties seeking medical attention and subsequently report PC. Yet, the results obtained from this study will add further information by analyzing suspected genetic factors, namely PC, in adult with epilepsy, and eventually help in understanding the genetics and the pathogenesis of epilepsy.
5. Conclusions
- In patients with epilepsy, the presence of PC appeared to have no effect on epilepsy etiologies or semiologies, and didn’t alter the EEG or MRI findings of these cases. The presence of FHE was significantly associated with PC, likely because of clustering of cases of epilepsy among these families. The multifactorial etiologies leading to epilepsy may justify the negative influence exerted by the presence of PC on epilepsy in the present study.
ACKNOWLEDGEMENTS
- I would like to thank Prof. Adel Bondok, Professor of Neuroscience for revising the manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML