-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2014; 4(1): 23-33
doi:10.5923/j.ajmms.20140401.05
Insulin Sensitizing Effect of Omentin Improves Glucose and Lipid Homeostasis in Diabetic Rats
Husam M. Edrees
College of Medicine-Department of Physiology- Zagazig-University, College of Public Health and Health Informatics, Qassim University, Saudi Arabia
Correspondence to: Husam M. Edrees, College of Medicine-Department of Physiology- Zagazig-University, College of Public Health and Health Informatics, Qassim University, Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Omentin is a novel adipokine produced preferentially by visceral adipose tissue. Omentin was recently shown to be involved in the development of obesity-associated insulin resistance and type 2 diabetes mellitus in human and animal models. The aim of this research is to identify the effect of omentin administration on glucose and lipid metabolism in experimental animals both in normal conditions and in experimentally induced diabetes mellitus type 1 and type 2. Design: A total number of 48 healthy adult male albino rats were used to study the effect of daily intraperitoneal (i.p) injection of omentin in a dose of 8 μg/Kg for 7 days on some metabolic parameters including glucose and lipid metabolism in experimental animals. Results: The results of this study showed that the mean values were found to be non-significant (P > 0.05) for all parameters on treatment with Omentin in both normal conditions when compared with control group and in Streptozotocin (STZ) induced Diabetic group when compared with that of STZ Induced Diabetic group. In STZ Induced Diabetic Insulin&Omentin Treated group, it was found that there were significant decrease in levels of Serum glucose, HOMA-IR, cholesterol, LDL cholesterol and Serum Triglycerides and significant increase in HOMA β and HDL cholesterol when compared with that of STZ Induced Diabetic group treated with Omentin only and of STZ Induced Diabetic group treated with insulin only. In addition, in High Fat Diet (HFD)-fed diabetic group – Omentin Treated), the mean values were found to be significantly lower for Serum glucose level, HOMA-IR, cholesterol level, LDL cholesterol level and Serum Triglycerides level and significantly higher for HOMA β and HDL cholesterol level with no significant change in Serum insulin when compared with that of HFD-fed diabetic group - control group). No significant change in BMI in all studied groups. Conclusion:The results of this studysuggest that omentinimproves glucose and lipid metabolism in the pathological diabetic conditions (type 1 DMand type 2 DM) with no significant effect on anthropometric measures in both types.Accordingly, insulin-mimetic, insulin-like, or insulin sensitizer effect of omentin might be assumed.
Keywords: Omentin, Diabetes, Insulin Resistance, Glucose Metabolism, Lipid Metabolism
Cite this paper: Husam M. Edrees, Insulin Sensitizing Effect of Omentin Improves Glucose and Lipid Homeostasis in Diabetic Rats, American Journal of Medicine and Medical Sciences, Vol. 4 No. 1, 2014, pp. 23-33. doi: 10.5923/j.ajmms.20140401.05.
Article Outline
1. Introduction
- The manifestations of diabetes cause considerable human suffering and enormous economic costs. Both acute and late diabetic complications are commonly encountered. Long- term complications represented by cardiovascular diseases, cerebrovascular accidents, end-stage renal disease, retinopathy and neuropathies are already major causes of morbidity, disability and premature death. The development of long-term complications is influenced by hyperglycaernia. Poor control of diabetes accelerates their progression. The incidence of obesity is rising rapidly in industrialized and developing countries. Increased abdominal visceral fat is associated with insulin resistance, type 2 diabetes and coronary heart disease[1]. In the last decade, numerous studies have revealed that adipose tissue secretes a variety of bioactive substances that could explain the relationship between visceral fat mass and increased metabolic risk including metabolic syndrome. These substances, termed adipokines or adipocytokines, include leptin, adiponectin, resistin, lipocalin 2, plasminogen activator inhibitor-1, interleukin 6, omentin and various growth factors[2]. Diabetes mellitus type 1 (also known as type 1 diabetes, or T1DM; formerly insulin dependent diabetes or juvenile diabetes) is a form of diabetes mellitus that results from autoimmune destruction of insulin-producing beta cells of the pancreas. The subsequent lack of insulin leads to increased blood and urine glucose. Untreated, type 1 diabetes is ultimately fatal[3].Omentin-1 is a novel 34 kDa adipokine, consisting of 313 amino acids. Omentin consists of two isoforms in which omentin-1 appears to be the major isoform in human plasma. It is preferentially produced by visceral adipose tissue compared with subcutaneous adipose tissue. It was also identified in human epicardial fat[4,5,6]. Omentin has been identified in other tissues at lower expression levels and named intelectin, intestinal lactoferrin receptor, or endothelial lectin. This adipokine may act at distant sites, e.g., muscle, liver and subcutaneous fat to enhance insulin sensitivity and glucose metabolism, and thus may play a wider role in nutrient storage and partitioning[7,8].Changing the focus to the in vitro studies, addition of recombinant omentin-1 did not affect basal but enhanced insulin-stimulated glucose uptake and protein kinase B (Akt) phosphorylation in both subcutaneous and omental human adipocytes[9]. Chronic administration of omentin increases body weight. The orexigenic effects of omentin-1 could be related, at least in part, to decreased cocaine and amphetamine-regulated transcript (CART) and corticotrophin releasing hormone (CRH) gene expression and increased nor epinephrine (NE) synthesis and release in the hypothalamus[10,11].Furthermore, studies in isolated rat aorta found that omentin directly induces an endothelium-dependent relaxation by nitric oxide (NO). Omentin is even capable of inducing vasorelaxation in rat mesenteric arteries, indicating the effectiveness of omentin in resistance vessels[12,13]. As regard the regulating mechanisms, circulating omentin-1 correlated negatively with body mass index (BMI), leptin level, waist circumference, fasting insulin and homeostasis modeling assessment of insulin resistance (HOMA). On the other hand, it is correlated positively with adiponectin and high density lipoproteins (HDL) in omentum[14]. Both subcutaneous adipose tissue (SAT)-released and plasma levels of omentin-1 were significantly lower in metabolic syndrome (MetS) compared to controls, and the significant differences persisted after adjustment for age, body mass index, or waist circumference. Omentin correlated significantly with high-density lipoprotein cholesterol and inversely with glucose and triglycerides. It was found that omentin-1 protein was secreted into the culture medium of omental, but not subcutaneous fat[15, 16].The ability of omentin to reduce insulin resistance in conjunction with its anti-inflammatory and anti-atherogenic properties makes it a promising therapeutic target. Thus, omentin may have beneficial effects on the metabolic syndrome and could potentially be used as a biologic marker and/or pharmacologic agent/target in this respect. The evidence to date indicates that omentin is an important endocrine peptide that links the metabolic system and adipose tissue in the regulation of glucose and lipid homeostasis and energy balance[17]. Because the effect of omentin on glucose and lipid homeostasis is unknown, so that this study was designed to investigate the role of omentin in metabolic homeostasis in different conditions. Therefore, the aim of this study is to clarify the in-vivo effects of omentin administration on glucose and lipid metabolism in experimental animals both in normal conditions and in experimentally induced diabetes mellitus.
2. Material and Methods
2.1. Experimental Animal
- The current study was carried on 48 adult male albino rats 12 – 15 weeks old with body weight 180-200 gm. They were obtained from the animal house from faculty of veterinary medicine, Zagazig University. Rats were kept in steel wire cages (6/cage) in the physiology research laboratory in faculty of medicine, Zagazig University under hygienic conditions. Rats were kept on the normal diet, which consisted of mixed commercial rat laboratory chow and supplied in separate clean containers. Animals had free access to water from graduated tanks, kept at room temperature and were maintained on a 12 hr light/dark cycle. All the animals were breed in the animal house and they fed the same type of food to avoid the effect of different food elements on the experiments[18]. The rats were accommodated to laboratory conditions for two weeks before the experiments going on. Rats were numbered and divided into the following groups:Group I: (Control group- n = 6): rats were served as a control group; they were fed on normal diet. Rats are injected intraperitoneally with distilled water for injection (0.1 ml) once daily at 1.30 PM for 7 days.Group II: (Omentin treated group- n = 6): rats were fed on normal diet. They were injected intraperitoneally with omentin-1 (8 μg/Kg) once daily[19] at 1.30 PM for 7 days.Group III: (Streptozotocin (STZ) induced diabetic group- n = 24): Experimental diabetes was induced by intra peritoneal injection of freshly prepared solution of streptozotocin (sigma) 65 mg/kg of body weight dissolved in 0.2 mmol/L sodium citrate, at PH 4.5 and maintained for 30 days[20,21]. To avoid incidental puncture of the intestines, which would have decreased the success rate for diabetes induction, all animals were fasted overnight. Three days later, diabetes induction was confirmed through measurement of blood glucose level in each animal (from blood sampled from the tail vein) with the One Touch Ultra Glucometer. Rats with diabetes (blood glucose > 8.88 mmol/L or 160 mg/dl) were selected for experiments[22, 23]. Then, diabetic rats were subdivided into:Group IIIa: (Diabetic untreated (control) group-n = 6): In which rats were neither treated by omentin nor insulin.Group IIIb: (Diabetic omentin treated group- n = 6): In which rats after ensuring their diabetes were treated with omentin-1 by intraperitoneal injection (dose = 8 μg/Kg)[19].Group IIIc: (Diabetic insulin treated group- n = 6): In which diabetic rats were treated with insulin (Humulin 70/30) by subcutaneous injection twice daily, in a dose designed according to blood glucose level that is measured before injection.Group IIId: (Diabetic omentin and insulin treated group- n = 6): In which diabetic rats were treated with both, omentin-1 (8 μg/Kg) once daily[19] in addition to insulin which injected subcutaneously according to blood glucose level twice daily.Group IV: High fat diet induced diabetic obese group (n = 12): In which rats were fed on high fat diet generally contain protein 20%, carbohydrates 35% and fat 45%. This fat mainly in form of lard and soya bean[24]. The rats were fed for 12 week till time of the experiment in a constant temperature (20℃ – 25℃) and humidity (50% - 60%) in animal room, with 12/12 hours light/dark cycles[25]. Blood glucose level was measured by a One Touch Ultra Glucometer using samples from tail vein after reaching body weight ranged from 350 – 460 gm (BMI range 0.72 – 0.95). Diabetes induction was confirmed through measurement of blood glucose level in each animal (from blood sampled from the tail vein) with the One Touch Ultra Glucometer. Rats with obesity BMI > 0.68 and diabetes (blood glucose > 8.88 mmol/L or 160 mg/dl)[22,23] were selected for experiments. Then, this diabesity group was subdivided into two groups:Group IV a: (Type 2 DM untreated group - n = 6): In which rats were injected intra-peritoneally with distilled water for injection (0.1 ml) once daily at 1.30 PM for 7 days.Group IV b: (Type 2 DM Omentin treated group - n = 6): In which rats were treated with omentin only by intraperitoneal injection (dose = 8 μg/Kg)[19] once daily at 1.30 PM for 7 days.For all groups, daily recording of food intake, water intake and body temperature were done at 1 PM. After one week, measures were done for body weight, other anthropometric measures at 1 PM. at the end of the study period.
2.2. Drugs and Chemicals
2.2.1. Omentin
- omentin recombinant from E.coli. BioVision - Analytica, catalog No. 4925 – 10. Product of USA.
2.2.2. Glucose (Anhydrous)
- ADWIC Laboratory Chemicals, Egypt.
2.2.3. Streptozotocin,[N-(Methylnitrosocarbamoyl)- α-D-glucosamine]
- (SIGMA-ALDRICH Co.-USA).
2.2.4. Insulin (Humulin)
- [70/30 100 iu/ml, 70% isophane insulin and 30% soluble insulin of rDNA origin]: (LILLY Egypt under license of ELI LILLY CO – USA).
2.3. Sampling of Blood
- At the end of the experimental period and after overnight fasting, at 8:00a.m, the animals were sacrificed to get a blood sample for centrifugation and analysis of glucose, insulin, total cholesterol, HDL and triglycerides with calculation of LDL cholesterol level [total cholesterol-HDL- (triglycerides/5)]. Also, measurement of homeostasis model assessment (HOMA), as a measure of insulin resistance [HOMA-IR = insulin (µU/mL) x glucose (mmol/L) / 22.5] and β-cell function[HOMA- β = 20 x insulin (µ U/mL) / (glucose - 3.5)][26].Blood samples were allowed to clot for 2 hours at room temperature before centrifuging for 20 minutes at approximately 500 rpm[27]. The separated serum was stored at -20℃. Repeated freezing and thawing was avoided[28].
2.4. Anthropometric Measures
2.4.1. Measuring Body Weight
- By using a digital scale sensitive down to one tenth of the gram. The animal was put in closed plastic container and was weighed day before the experiment, every day and at the last day[24].
2.4.2. Measuring Rat Length
- Nose to anus length was measured at the start and the end of the experiment. The animal is allowed to move while an assistant was holding it from the tail to lengthen the body, ensure the real nose to anus length of the animal and avoid false measures. Metal ruler graduated in centimeters was used by holding zero end at the anus and record the reading that reached by the nose, then all measures were plotted for each labeled rat in its record[29].
2.4.3. Calculating Body Mass Index
- (BMI: By using the previous data we can calculate body mass index (BMI) which equal body weight (gm) / length2 (cm2), this index can be used as an indicator of obesity where the cutoff value of obesity BMI is more than 0.68 gm/cm2[29].
2.5. Laboratory Analysis
2.5.1. Estimation of Serum Glucose Level
- According to Trinder[30] using glucose enzymatic (GODPAP) - liquizyme Kits (Biotechnology, Egypt).
2.5.2. Estimation of Serum Insulin Level
- By a solid phase enzyme amplified sensitivity immunoassay according to Starr et al.[31] using KAP1251-INSEASIA (Enzyme Amplified Sensitivity Immunoassay) Kits (BioSource Europe S.A., Belgium).
2.5.3. Determination of Insulin Resistance (HOMA-IR)
- It was assessed by homeostasis model assessment (where HOMA-IR= (fasting insulin (μU/ml) x fasting plasma glucose (mg/dl) /405.
2.5.4. Determination of β-cell Function (HOMA- β)
- It was assessed by homeostasis model assessment (where HOMA- β = 20 x insulin (µ U/mL) / (glucose - 3.5)][26].
2.5.5. Estimation of Serum Total Cholesterol (TC) Levels
- Cholesterol was estimated by enzymatic colorimetric method according to Allain[32] using Cholesterol RTU 61218 kits: (bioMerieux S.A., Lyon, France).
2.5.6. Estimation of the Serum High Density Lipoprotein Cholesterol (HDL-C)
- by enzymatic colorimetric method according to Warnick et al,[33] using Stanbio HDL-cholesterol procedure No. 0599 kits (Stanbio laboratory Inc., San Antonio, Texas, USA).
2.5.7. Calculation of Low Density Lipoprotein Cholesterol (LDL-C)
- According to Friedewald et al.[total cholesterol-HDL- (triglycerides/5)][34].
2.5.8. Estimation of Serum Triglycerides
- It was carried out according to Naito[35] using triglycerides ESPAS SL kits (Elttech S.A., Sees, France.).
2.6. Statistical Analysis
- Results are presented as mean ± standard error of the mean. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 17.0 (SPSS Inc., Chicago, IL, United States). Repeated measures of analysis of variance (ANOVA) statistical analysis were applied followed by the Student-Newman-Keuls post hoc test. P value <0.05 was considered to be statistically significant.
3. Results
- Table 1 and histograms 1, 2, 3 & 4 show Body Mass Index (BMI), Serum glucose (mmol/L), Serum insulin (μIU/ml), HOMA index of insulin resistance (HOMA-IR), HOMA index of β Cell Function (HOMAβ), in all studied groups. It was found that the mean values for BMI were found to be non-significant (P > 0.05) in all groups. In group II (Omentin Treated), the mean values were found to be non-significant (P > 0.05) for all parameters when compared with that of group I (control group).
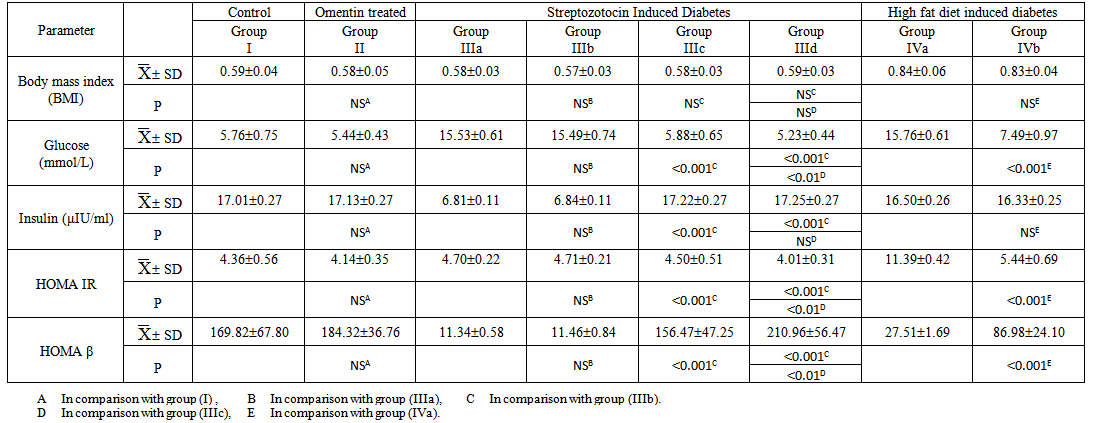 | Table (1). The Laboratory Data (BMI, Serum Glucose, Serum Insulin, HOMA IR and HOMA B) in all studied Groups |
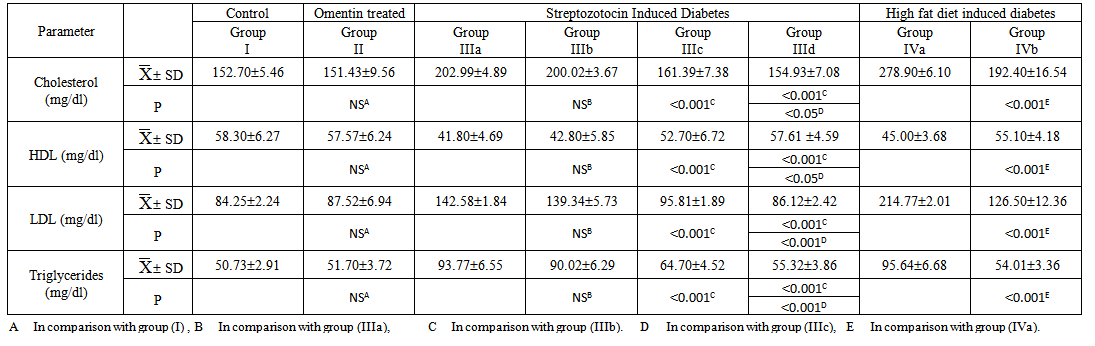 | Table (2). The Laboratory Data (Serum Cholesterol, Serum HDL, Serum HDL and Serum Triglycerides) in all studied Groups |
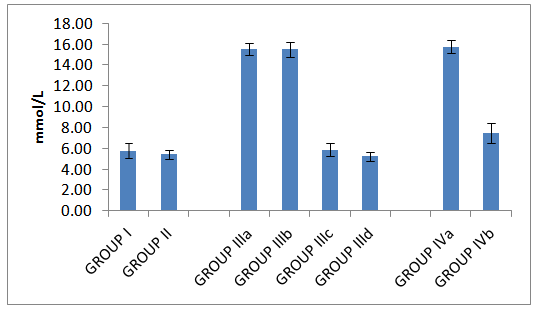 | Histogram 1. Serum glucose in all studied groups |
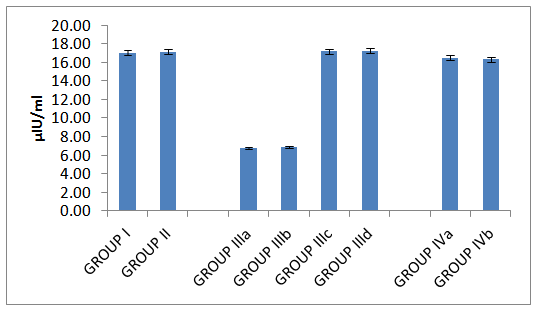 | Histogram 2. Serum insulin in all studied groups |
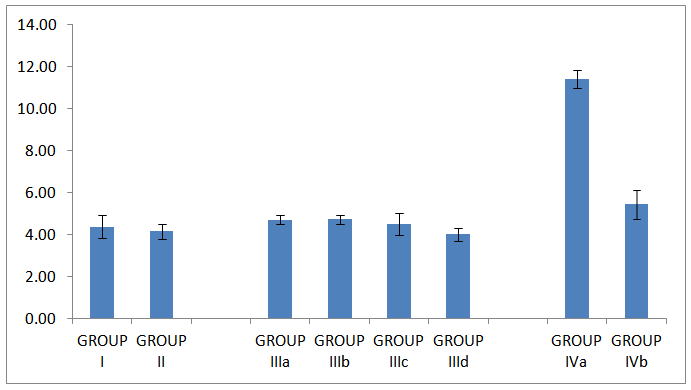 | Histogram 3. HOMA IR in all studied groups |
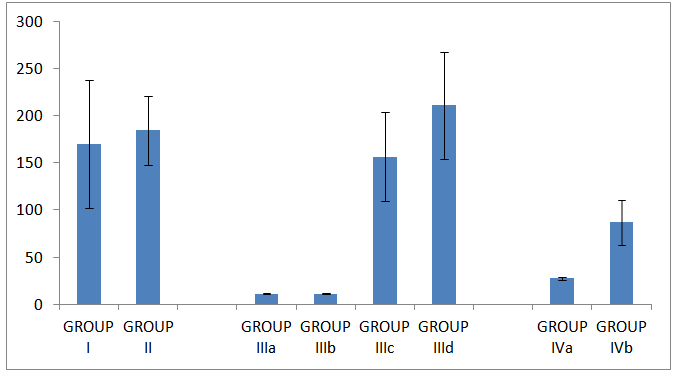 | Histogram 4. HOMAβ in all studied groups |
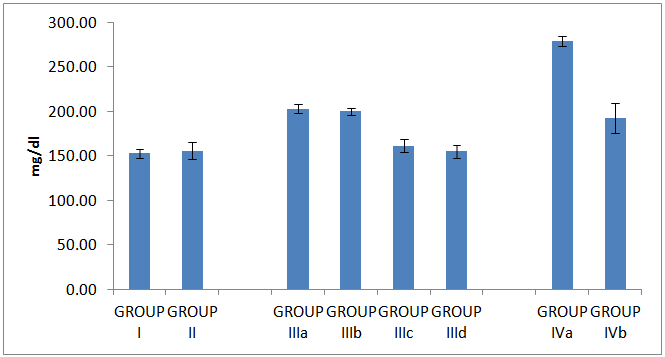 | Histogram 5. Serum cholesterol in all studied groups |
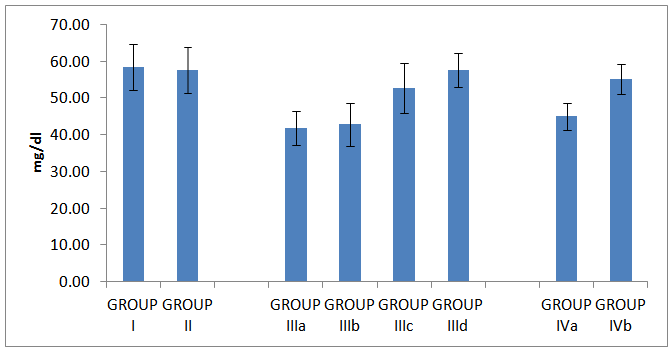 | Histogram 6. HDL in all studied groups |
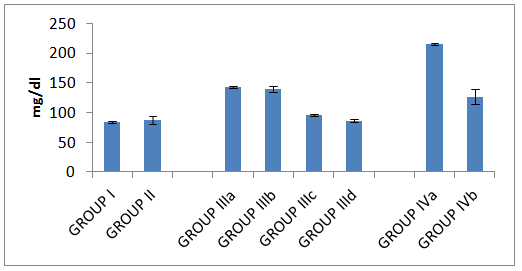 | Histogram 7. LDL in all studied groups |
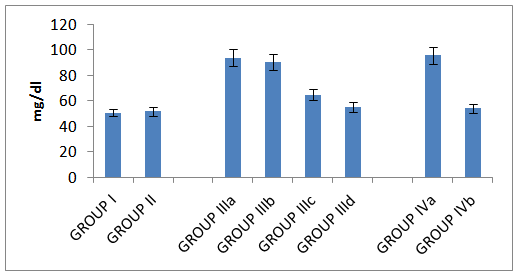 | Histogram 8. Level of Triglycerides in all studied groups |
4. Discussion
- Omentin-1 (intelectin-1) is a newly identified adipokine that is highly and selectively expressed in visceral adipose tissue[36]. The results of the present study concerning body mass index revealed that there was a non-significant change in all groups treated with omentin either in Streptozotocin (STZ) induced diabetic group or High fat diet induced diabetic obese group. These insignificant changes may be attributed to the short term injection of omentin (7 days). This result is in good accordance with Lesná et al.[37] and Hossein-nezhad et al.[38] who revealed that plasma omentin levels during the short term control of body weight were stable, but increased significantly after a year of the regime changes with no relation was present between omentin and BMI as an indicator of obesity. Another supporting study done by Brunetti et al.[39,40] who reported that omentin-1 significantly increased food intake since day 10 of treatment (days 10-13, P<0.05; day 14, P<0.01) and body weight on day 14 (P<0.05). Omentin-1 treatment led to a significant reduction in both anorexigenic cocaine- and amphetamine-regulated transcript (CART) peptide (P<0.05) and corticotropin releasing hormone (CRH) (P<0.05) gene expression, while POMC, AgRP, NPY and orexin-A gene expression were not modified with respect to vehicle-treated rats. In hypothalamic synaptosomes, omentin-1 (10-100 ng/ml) stimulated basal NE release. They suggested that neither chemerin nor omentin-1 showed any acute effect on food intake and despite their correlations with fat mass and glucose homeostasis; do not affect feeding behavior and so body mass index (BMI) in the tested dose. Contradictory results were shown by Brunetti et al.[10], Moreno-Navarrete et al.[41] and Catli et al.[42] who showed that chronic administration of omentin increases body weight. The orexigenic effects of omentin-1 could be related, at least in part, to decreased cocaine and amphetamine-regulated transcript (CART) and corticotrophin releasing hormone (CRH) gene expression and increased nor epinephrine (NE) synthesis and release in the hypothalamus. They observed an increase in circulating omentin-1 concentration after weight loss and its association with the improvement in insulin sensitivity and negative correlation between omentin 1 level with BMI in the obese group.To go through the possible mechanism of lowering serum levels of glucose, this study investigated the homeostatic model assessment for insulin resistance (HOMA IR) and β cell function (HOMA β). An impressing finding of the study was the decreased insulin resistance (a significant decrease in serum glucose level, HOMA-IR ) with increased β cell function (a significant increase in HOMA β ) in Streptozotocin (STZ) induced diabetic group treated with insulin and omentin when compared with STZ induced diabetic group treated with omentin only or the group treated with insulin only. Moreover, treating the high fat diet induced diabetic obese group with omentin results in significant decrease of serum glucose level and insulin resistance with increase insulin sensitivity with no effect on insulin level itself. These finding could suggest the important role of omentin-insulin-sensitizing effect in Streptozotocin (STZ) induced diabetes and the high fat diet induced diabetic obese group. Accordingly, insulin-mimetic, insulin-like, or insulin sensitizer effect of omentin might be assumed.These results were in agreement with the insulin-mimetic effect of omentin showed by Jaikanth et al. and Greulich et al.[43,44] who found that, plasma omentin-1 levels were significantly decreased versus controls in patients with obesity and diabetes that contribute to the major components of the metabolic syndrome and other disease conditions like atherosclerosis and autoimmune disorders and concluded omentin-1 as a cardioprotective adipocytokine. Derosa et al.[45,46] showed decreased insulin resistance with increased β cell function in the obese/diabetic groups after injection of omentin. This speculated anti-diabetic property of omentin was shown by many study groups but for different oral anti-diabetic agents.Daozhan Yu[47] reported that Omentin is decreased with obesity and is inversely correlated to insulin resistance. It has been shown that omentin activates AKT (Protein Kinase B) and increases insulin-mediated glucose uptake in adipocytes indicating that omentin may serve as an insulin sensitizer. Omental fat has less insulin receptors and higher lipolysis in comparison to subcutaneous fat. It has been proposed that free fatty acids that released from visceral adipose tissue can decrease insulin clearance and increase gluconeogenesis by liver. If omentin serves as an insulin sensitizer, it would inhibit lipolysis and have beneficial functions in insulin resistance. This is important especially because as an insulin sensitizer, adiponectin, is expressed less in omental fat where omentin could compensate it in maintaining fatty acid storage and mobilization. Activation of AMP-activated protein kinase (AMPK) by adiponectin has been shown to inhibit lipolysis. He found that omentin also increases AMPK phosphorylation in adipocytes, suggesting that omentin may inhibit lipolysis. Omentin’s correlation to obesity and its function in energy metabolism resemble adiponectin except that omentin is expressed in stromal vascular cells rather than in adipocytes. This could be of advantage in evolution under some condition, such as in omental fat, where adiponectin expression is inhibited while omentin from stromal vascular cells can still fulfill its function in energy metabolism. This could also be true during infection when adiponectin expression is decreased while omentin is increased, so the host can still maintain energy homeostasis.In conjunction with glycemic control, lipid profile stands in the same priority. The results of the current study showed that there was a non-significant change in lipid profile in Streptozotocin (STZ) induced diabetic group treated with omentin only, however, treating Streptozotocin (STZ) induced diabetic group with insulin and omentin significantly reduced serum level of cholesterol, LDL cholesterol and Serum Triglycerides and significantly increased serum level of HDL cholesterol. On the other hand, treating the high fat diet induced diabetic obese group with omentin results in a significant decrease in serum level of cholesterol, LDL cholesterol and Serum Triglycerides and a significant increase in level of HDL cholesterol adding more beneficial effects of omentin as insulin sensitizer on lipid profile improving dyslipidemia which is present in high fat diet induced diabetic obese group.Jialal et al. and Shibata et al. [48,49] showed that omentin correlated significantly with high-density lipoprotein cholesterol and inversely with glucose and triglycerides. Furthermore, a reduction of plasma omentin levels significantly correlated with an increase in the mean number of metabolic risk factors such as increased waist circumference, dyslipidemia, high blood pressure and glucose intolerance. Circulating omentin levels negatively correlated with the multiplicity of metabolic risk factors, suggesting that omentin acts as a biomarker of metabolic disorders. Contradictory results obtained by Hossein-nezhad et al.[38] who found that Levels of omentin-1 were lower in obese than non-obese subjects. It was also lower in patients with metabolic syndrome than in healthy ones. In correlation analysis, omentin-1 was associated with waist circumference, fat percent and fat mass and nearly visceral fat. But there was no significant relation between omentin and any insulin resistance indices. Regarding the lipid metabolism, Yu and Herder et al.[50,51] showed that omentin activates AMPK and play a role in energy metabolism. AMP-activated protein kinase (AMPK) acts as a metabolic sensor of the cellular energy status. It is activated by phosphorylation of its Thr172 residue and its activation inhibits the rate-limiting enzymes in cholesterol and fatty acid synthesis, 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase and acetyl CoA carboxylase (ACC) which saves ATP. AMPK-activation promotes fatty acid oxidation and glucose uptake in myocytes, reduces gluconeogenesis in hepatocytes, and inhibits lipogenesis in adipocytes.
5. Conclusions
- The present study revealed that omentin injection combined with insulin injection in Streptozotocin Induced Diabetes and omentin injection in the high fat diet induced diabetic obese group improved insulin resistance, insulin sensitivity and dyslipidemia. These findings could suggest an important role of omentin as insulin-sensitizer adding more beneficial effects of omentin on glucose and lipid homeostasis especially in diabetic conditions. Interestingly, short term omentin injection did not affect body mass index in all studied groups compared with its effect in chronic administration revealed in other studies. Additional researches are needed to clarify the role of omentin, omentin receptors and their mechanisms of action in pathogenesis of insulin resistance and obesity.
ACKNOWLEDGMENTS
- The author would like to acknowledge the supporting team of technicians in the Department of Physiology and Medical Biochemistry, Zagazig University for their assistance in the experimental work and laboratory analysis. This research received no specific grant from any funding agency in the public commercial or not-for-profit sectors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML