-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2013; 3(6): 140-145
doi:10.5923/j.ajmms.20130306.05
Efficacy of Solanum Tuberosum in Reducing Food Intake and Fasting Blood Glucose Level in the Management of Body Weight
T. H. Olubobokun 1, E. O. Aluko 1, V. U. Nna 2, E. D. Olatunbosun 3, D. E. Atang 1
1Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Akwa Ibom State, Nigeria
2Department of Physiology, Faculty of Basic Medical Sciences, University of Calabar, Calabar, Cross River State, Nigeria
3Department of Medical Biotechnology, University Pecs, Pecs, Hungary
Correspondence to: T. H. Olubobokun , Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Akwa Ibom State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Stabilization of hunger control, blood sugar and preservation of lean body mass and metabolism seems to be the most effective way to lose weight and improve glucose control in people with obesity. This study was therefore designed to determine the effect of Solanum tuberosum on food intake, fasting blood glucose level and subsequent effect on body weight with a view to recommend it as a diet for the obese population. The phytochemical constituents and median lethal dose of the plant material were determined before administration. Twenty four male wistar rats weighing 170-180g were randomly assigned into one of four groups such that each group contained six animals. Group 1 served as control while groups 2 - 4 served as the test groups and were given the plant extract at a dose of 100, 200 and 300 mg/kg body weight respectively. The extract was given once daily per oral route. Food intake, fasting blood glucose level and body weight were measured throughout the study which lasted for 21 days. Solanum tuberosum significantly (P<0.05) reduced food intake, fasting blood glucose level and body weight compared to control. The decrease was most significant in the group administered 300mg/kg body weight of the extract. Results also showed that the effect of Solanum tuberosum extract was time dependent as the most significant (P<0.05) decrease were observed in the third week compared to the first week. Solanum tuberosum can serve as a diet in the management of food intake and body weight.
Keywords: Blood glucose, Body weight, Food intake, Obesity, Solanum tuberosum
Cite this paper: T. H. Olubobokun , E. O. Aluko , V. U. Nna , E. D. Olatunbosun , D. E. Atang , Efficacy of Solanum Tuberosum in Reducing Food Intake and Fasting Blood Glucose Level in the Management of Body Weight, American Journal of Medicine and Medical Sciences, Vol. 3 No. 6, 2013, pp. 140-145. doi: 10.5923/j.ajmms.20130306.05.
Article Outline
1. Introduction
- Restriction of energy intake was believed to be the most effective way to lose weight and improve glucose control in people with obesity and diabetes[1], therefore obesity was normally treated by diet and exercise, but attempts to sustain significant weight loss by lifestyle intervention often fail[2] because this method also reduces satiety and increases appetite, which makes adherence to an energy- restricted diet difficult[3]. Stabilization of blood sugar, hunger control, and preservation of lean body mass and metabolism is important[3]. Numerous biopsychological factors affect our eating behavior. The arcuate and paraventricular nuclei in the ventromedial hypothalamus[4] are parts of a system integrating body composition with energy intake and expenditure[5]. Obesity can occur when energy intake exceeds energy expenditure. There has been considerable interest in dietary composition[6] and weight fluctuations[7]. The effects of diet composition on the development of obesity can be clearly seen in animal models[8]. Obesity is rare in experimental animals maintained on a low-fat diet, even when they are housed in small cages that limit physical activity. In contrast, providing sedentary animals with ad libitum high-fat diets, reliably produces increase in energy intake, increase in efficiency of body fat gain, and obesity[9]. Our environment provides an unlimited supply of convenient, highly palatable, non-satisfying energy-dense foods, coupled with a lifestyle requiring only low levels of physical activity and this promotes high energy intake and low energy expenditure[8]; thereby increasing the prevalence of obesity.Appetite management is one approach to moderate food intake; energy intake is determined by the amount or size of energy consumed (satiation) and the frequency (satiety) of consuming the food[10]. Attempts to moderate intake by focusing only on satiation or satiety may be unproductive because of compensation by the other[11]. Numerous food components have been explored for their satiation/satiety value. Dietary fiber is one constituent with well documented effects[12,13] on satiation, satiety and reduction of energy intake[14]. Its characteristics such as high viscosity[15], orosensory stimulation, greater mastication time[16], ability to increase gastric distension; prolong gastrointestinal transit time, slow nutrient absorption [17] and its effect on some gastrointestinal hormones that influence food intake[18] depicts this. Dietary fibers have also been shown to improve blood lipid levels, regulate blood glucose, and increase satiety, which may help with weight loss[19]. The potato is a starchy, tuberous crop from the perennial Solanum tuberosum of the Nightshade family. Humans can survive healthily on a diet of potatoes supplemented only with milk or butter, which contain the two vitamins not provided by potatoes (vitamins A and D)[20]. The fiber content of a potato with skin (2 g) is equivalent to that of many whole grain breads, pastas, and cereals. One medium potato with the skin contributes two grams of fiber or eight percent of the daily value. Potato is best known for its carbohydrate content; the predominant form of this carbohydrate is starch[3] which is resistant to enzymatic digestion in the small intestine[3]. The amount of resistant starch in potatoes depends much on preparation methods. Cooking and then cooling potatoes significantly increases resistant starch[21,22]. Its systemic effects include improvements in glucose tolerance and insulin sensitivity, reductions in blood lipid levels, increases in satiety and potential uses in weight management[23,24].Potatoes have been hypothesized to yield a relatively high glycemic response but glycemic index (GI) is not an inherent property of a food but, rather, the metabolic response of an individual to a food[25]. Also the dietary fibre and resistant starch in potato could counteract the effects related to the glycemic response, with regard to hunger and food intake. Hill et al[8] also reported that one way to improve dietary adherence rates may be to enhance satiety through the use of protease inhibitors. Potato tuber is the source of potato protease inhibitor II (PI-2), which is active in eliciting a satiety response[8] and delayed gastric emptying in humans [26]. It was suggested that PI-2 promotes satiety by increasing circulating levels of cholecystokinin (CCK) similar to soybean trypsin inhibitor;[27]. CCK has been shown to reduce food intake and elicit behaviors associated with satiety in mammals[28,29].Data suggest that the 2-year persistence rate with orlistat or sibutramine, the only Food and Drug Administration (FDA) - approved drug therapies for obesity, does not exceed 2%.[30]. Therefore, novel therapeutics to reduce food intake and body weight with minimal adverse reactions is highly desirable. This study was therefore designed to determine the effect of Solanum tuberosum on food intake, fasting blood glucose level and subsequent effect on body weight.
2. Material and Methods / Experimental Details
2.1. Plant Material and Preparation of Extract
- Fresh Solanum tuberosum weighing between 50-60g were purchased locally from the Ogbette main market, Enugu State, Nigeria. The plants were subsequently identified and authenticated by a Botanist of the Botany Department of the University of Nigeria, Nsukka, Nigeria. The tubers were chopped into small pieces and homogenized in distilled water (0.25g tissues/mL of water) for 30seconds using a fisher scientific tissuemiser portable homogenizer (Healthcare, Lab & Life Science San Diego, California, United States). Homogenate was filtered through muslin cloth with a mesh size of 2 mm into centrifuge tubes and then centrifuged at 120rpm for 20 minutes. For use, the residue was evaporated to dryness. The dried extract was reconstituted in freshly prepared normal saline (1g of extract in 10ml of normal saline) for administration to test animals. Extract was stored in capped tubes and refrigerated until when required for use. Plant extraction was carried out according to the method of Al-salkhan et al[31] but with some modifications.The phytochemical constituents of Solanum tuberosum showed that it contains dietary fibers, vitamins and minerals[32], carotenoids and natural phenols[33,34]. Chlorogenic acid constitutes up to 90% of the potato tuber natural phenols. Others found in potatoes are4-O-caffeoylquinic acid (crypto-chlorogenic acid), 5-O-caffeoylquinic (neo-chlorogenic acid), 3,4-dicaffeoylquinic and3,5-dicaffeoylquinic acids[35]. The USDA[36] (Nutrient data laboratory) stated that nutritional value per 100g (3.5oz) of potato contains the following: ● Carbohydrates -17.47 g; Starch - 15.44 g Dietary fiber - 2.2 g● Fat - 0.1 g ● Protein - 2 g● Water - 75 g● Vitamins and minerals: The Vitamins are: Thiamine (vit. B1) - 0.08 mg (7%), Riboflavin (vit. B2) - 0.03 mg (3%), Niacin (vit. B3) - 1.05 mg (7%), Pantothenic acid (B5) - 0.296 mg (6%), Vitamin B6 - 0.295 mg (16%), Folate (vit. B9) - 16 μg (4%), Vitamin C - 19.7 mg (24%), Vitamin E - 0.01 mg (0%), Vitamin K - 1.9 μg (2%). The minerals are: Calcium - 12 mg (1%), Iron - 0.78 mg (6%), Magnesium - 23 mg (6%), Manganese - 0.153 mg (7%), Phosphorus - 57 mg (8%), Potassium - 421 mg (9%), Sodium - 6 mg (0%), Zinc - 0.29 mg (3%).
2.2. Animal Preparation, Experimental Groupings and Treatment
- Twenty four in-bred male wistar rats weighing 170-180g were used for this study. They were obtained from the Enugu campus Animal House, University of Nigeria. The animals were kept in a conducive, healthy environment for the period of the experiment in clean steel-gauzed cages. They were fed on standardized animal pellets (suppex starter fedR) and tap water ad libitum for two weeks for acclimatization to standard laboratory conditions before the experiment. Before the commencement of the experiment, the rats weighed averagely between 170 and 180g. An acute oral toxicity test was carried out before administration of the extract according to Lorke’s method[37] and it was found to be non toxic at 2000 mg/kg body weight. The animals were randomly assigned into 4 groups of 6 rats each; each rat in a group was individually caged in a cubicle in a larger cage. Group 1 served as the control and were fed with 0.3ml of normal saline, Group 2 - 4 were fed with ST extract at 100, 200 and 300mg/kg body weight (bwt) respectively.Administration of the aqueous extract was done before feeding by means of calibrated syringe with attached rubber cannula (1g of extract in 10ml of normal saline) by oral gavage. The experimental procedures involving the animals and their care were in line with the approved guidelines by the local research and ethical committee.
2.3. Measurement of Parameters
- Food intake was determined everyday by giving 100g of feed to all groups and the remaining quantity was measured the following day to determine the quantity eaten by each group[38]. Fasting blood glucose was determined after an overnight fasting and body weight was measured just before feeding the extract and further readings were taken every 7 days. Fasting blood glucose level and changes in body weight were measured on days 0 (initial reading), 7, 14 and 21. Fasting blood glucose level was measured using a glucometer (Life Scan Inc. milano, Italy), and the body weight was measured with a spring balance.
2.4. Statistical Analysis
- Data obtained were subjected to descriptive statistics and the results presented as mean ± standard error of mean (Mean ± SEM). Differences between means were separated by one-way analysis of variance (ANOVA), followed by post hoc multiple comparisons (Gabriel), with the least significant threshold employed at P≤0.05. Data analysis was done using the statistical software package SPSS for windows version 17.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. The Effect of ST Extract on Food Intake
- Table 1 showed the effect of ST on food intake of the various groups at the different concentrations. The result showed that the food intake of the test groups was significantly (P<0.05) lower than those of the control group. There was an inverse relationship between the extract doses and food intake. The food intake decreased as the extract dose increased. At 100 mg/kg, the food intake of the group was not significantly different from the control group but at 200 mg/kg it became significantly different and more significantly reduced at 300 mg/kg.
|
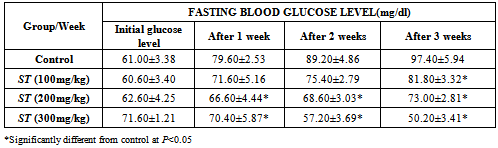
3.2. The Effect of ST Extract on Fasting Blood Glucose Level
- Table 2 shows the effect of ST extract on the fasting blood glucose level. Fasting blood glucose for rats ranges from 50 to 109 mg/dL. Therefore, the fasting blood glucose level of all the different groups still fall within the normal range. The initial glucose level was not significantly different from the control but over the weeks, the glucose level of the test groups became lower, having more significance (P<0.05) at 300 mg/kg compared to control.
|
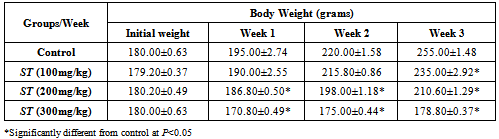
3.3. The Effect of ST Extract on Body Weight Changes
- Table 3 shows the mean body weight and weight changes of the various groups given ST extract and those of the baseline control. The initial body weight of all the groups was not significantly different but over the weeks, the body weight of the test groups became significantly (P<0.05) lower than the control group. There was more significant (P<0.05) weight loss at 300 mg/kg compared to control.
|
4. Discussion
- Restriction of food intake was believed to be the most significant way in managing body weight but this leads to hunger which reduces satiety and increases appetite, thus making adherence to an energy restricted diet difficult, sometimes leading to eating more than one used to or need. Excessive weight gain can be managed by appropriate adjustments of food intake and energy expenditure; stabilization of blood sugar, hunger control, and preservation of lean body mass and metabolism is important in the restriction of energy intake[3].Food intakeIn our study, we observed that oral intake of ST extract for 21 days reduced food intake, fasting blood glucose level and body weight (Table 1-3) significantly (P<0.05) when compared with the control group. The weight loss observed in the extract - treated groups might be due to reduced food intake and fasting blood glucose level. A reduced glucose level may help the body to use stored reserves from fat or muscle, gradually leading to weight loss.Oral intake of ST was able to cause a significant (P<0.05) reduction in food intake in the ST treated groups than the baseline control. The reduction in food intake seems to be dose dependent because at 100mg/kg bwt, the food intake of the ST group was not significantly different from those of the control group, but at 300mg/kg bwt, it caused a significant reduction in food intake in the group. The reduction in food intake also seems to be time - dependent because a more pronounced reduction in food intake was observed in the second week compared to the first week. Slavko et al[1] observed that Potato protease inhibitor concentrate (PPIC) reduced food intake during the first and second hour following oral administration in their study. Our findings are also consistent, these findings are consistent with the report of Little (2005)[39] who observed that the oral administration of a proteinase inhibitor 2 (PI2) has been shown to stimulate CCK release and reduce caloric intake. Hu et al (2004)[40] also observed that oral administration of PI2 was able to significantly decrease hunger in overweight and healthy subjects in their study. It has also been reported by Olubobokun et al[41] that administration of aqueous extract of Ipomoea batatas also significantly reduced food intake. Therefore the reduction in food intake observed might be due to presence of PI-2 in the extract which might probably reduce hunger in the rats thereby decreasing food intake. Dietary fiber have been explored for their satiation / satiety value; it may also affect some gastrointestinal hormones that influence food intake[18] and Solanum tuberosum have been discovered to contain a good percentage of it[36], therefore the crude extract administered which possibly still contained the dietary fibre could also cause the reduction in food intake by enhancing satiety. The predominant form of the carbohydrate in Solanum tuberosum is resistant starch[3] which is resistant to enzymatic digestion in the small intestine which has been observed to increase satiety[23,24], therefore the reduced food intake could also be as a result of the resistant starch.Fasting blood glucose levelAll rats used for this study had normal fasting blood glucose level; fasting blood glucose for rats is said to range from 50 to 109 mg/dl[42] and the fasting blood glucose level of rats used for this study ranged between 50 to 97 mg/dl. At the beginning of the study, the fasting blood glucose level of all groups were not significantly different from the control but at the end of the study, the glucose level of the various treated groups became significantly lower than the control group, though they all still fell within the normal range for a fasted rat. In table 2, ST was observed to cause a more significant reduction at 300 mg/kg which might be due to the reduced food intake observed at this dose which probably causes reduced plasma glucose. Schwartz et al[26] reported that their subjects showed a significant reduction in plasma glucose levels and plasma insulin levels when PI-2 was added to their meal. Also, Spreadbury et al[43] observed that when the meals of their subjects were supplemented with PI2, it modified their glycemic response. Their study showed a significant decline in postprandial blood glucose in patients treated with 15 and 30mg doses of PI-2 but no significant decline occurred in the group taking 7.5mg of PI-2. Our study also showed that there was no significant reduction in fasting blood glucose level in the group administered with 100 mg/kg of the extract in the first and second week. Viscous, water-soluble fibre such as β -glycans and pectin found in potato has also been reported to modify or reduce blood glucose response by interfering with digestion and absorption of glycemic carbohydrates[44]. It has been reported that aqueous extract of Ipomoea batatas reduced fasting blood glucose level in normal rats[41]. Dietary fibers have been shown to regulate blood glucose level (FNB); resistant starch has also been reported to improve glucose tolerance and insulin sensitivity[23,24]. Therefore, the reduction of the fasting blood glucose level could also be due to PI-2, dietary fibre or the resistant starch.Body weight changesOral administration of ST extract for 21 days reduced body weight gain in healthy rats. A significant reduction in body weight was observed in the extract - treated groups when compared with control group. At the onset of the study, the mean body weight of the treated groups were not significantly different from the control group but at the end of the study, a significant (P<0.05) reduction in body weight was observed in the treated groups compared to control.In Table 3, at 100 mg/kg of ST, there was no significant change between the weight gain of the test group and the control group, the weight of the test group was increasing almost at the same rate with the control group. At 200 mg/kg, ST still caused weight gain but significantly lower than the control and 100 mg/kg. The ST was able to cause a significant weight loss at 300 mg/kg, which might be due to reduced food intake and fasting blood glucose level observed in this group. Slavko et al[1] reported that oral intake of PPIC for 10 –14 days reduced the body weight gain in healthy rats and that the reduction can be partially explained by a decrease in cumulative food intake during the time of their experiment. Our study also support the report of Speigel et al[45], who reported that an average 2kg weight loss was observed in overweight women when PI-2 was taken daily prior to lunch and dinner for four weeks. This study is also consistent with the work by Dana[46] who reported that PI-2 was effective for weight loss and improved body measurements when taken before a meal by reducing appetite ratings and between meals snacking in human subjects. It is believed that PI-2 has been shown to induce CCK which then leads to satiety[46] and well documented weight loss. Dietary fiber have also been shown to improve blood lipid levels, regulate blood glucose, and increase satiety, which may help with weight loss[19] while resistant starch help improve glucose tolerance and insulin sensitivity, reduce blood lipid levels, increase satiety which could help in weight management[23,24]. Olubobokun et al[41] also observed that aqueous extract of Ipomoea batatas L. reduced body weight in healthy male wistar rats. This point to the fact that a food that increases short term satiety decreases the amount of energy ingested subsequently and thus could potentially help in weight management in the long run[48].Individually, three of the components of Solanum tuberosum which are PI-2, dietary fibre and resistant starch has been observed to reduce food intake either by enhancing satiety or stimulating some gastrointestinal hormones that influence food intake; they have been observed to modify glycemic response to a meal, regulate blood glucose level and improve glucose tolerance and insulin sensitivity respectively. They also have been observed to help in weight management by controlling food intake and regulating blood glucose level.
5. Conclusions
- Solanum tuberosum extract have been able to significantly reduce food intake, fasting blood glucose level and body weight gain. These effects might be a synergism between the PI-2, dietary fibre and resistant starch which have been individually reported to reduce food intake, regulate blood glucose and reduce body weight, therefore the observed effect might tend to be more pronounced by the potentiating effect of each of these component. Solanum tuberosum could therefore serve as a diet in the management of food intake and body weight.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML