-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2013; 3(6): 133-136
doi:10.5923/j.ajmms.20130306.03
Teratogenic Effects of Phenytoin on the Length of Bones of the Uper and Lower Extremities Development on Mice Fetuses (NMRI)
Bibi Mahboobeh Torabi1, Sepideh Shadab Shams Abad1, Abdolhosein Shiravi2
1Department of Biology, University Azad Islamic Damghan Unit, Damghan, Iran
2Department of Biology, University Azad Islamic , Semnan branch, Iran
Correspondence to: Bibi Mahboobeh Torabi, Department of Biology, University Azad Islamic Damghan Unit, Damghan, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Teratogenic effects of phenytoin on ossification of the bones of the upper and lower extremities on mice fetuses (NMRI). This study was desigened to determine the teratogenic effects of phenytoin on ossification of the upper and lower extremities on mice fetuses (NMRI). In this study used 40 white small pregnant mice (NMRI type) were divided in to three experimental groups (I,II,III) and one control group. Three experimental groups received 60,75 and 90 ml/kg/day of phenytoin solution in normal salin 0.2 cc volum from the day 6.5 (GD 6.5) to day 14.5 (GD 14.5) of pregnancy intraperitoneally (ip) and the control (group received normal saline with the same volume the mice on the eighteenth and a half day of pregnancy were sacrificial and the fetuses staind with Alizarin Red S/ Alcian blue and length of bones of the upper and lower extremities were measured with stereomicroscope that was equipped with a calibrate lens. Data were analysed by ANOVA T-test and tukey using SPSS software. Results showed that average of bones length of upper and lower extremities in three experimental groups were significauntly shorter compared with the control group (p>0.005). Also this study revealed that phenytoin can reduce ossifsication of the upper and lower organs if this drug is used subsequent to organogenesis stage .
Keywords: Phenytoin, Teratogenicity, Fetal, Mouse
Cite this paper: Bibi Mahboobeh Torabi, Sepideh Shadab Shams Abad, Abdolhosein Shiravi, Teratogenic Effects of Phenytoin on the Length of Bones of the Uper and Lower Extremities Development on Mice Fetuses (NMRI), American Journal of Medicine and Medical Sciences, Vol. 3 No. 6, 2013, pp. 133-136. doi: 10.5923/j.ajmms.20130306.03.
1. Introduction
- Teratology is the branch of embryology that studies the causes, mechanisms and patterns of abnormal development. deals. This is the fundamental issue in teratology is that specific stage of embryonic development are more vulnerable than other stages. Teratogenesis including functional and structural. Defects that can encompass the death of a baby before or shortly after birth . Functicnal and structural defects lead to permanent changes in a fetus within the womb exposed to a teratogen factor. Factor that could cause deformities are called teratogen. Some teratogen crosses the placental barrier and can affecte fetal period and may cause abnormality. However, these drugs cause improvement for the mother, but can cause toxic effects on the fetus and these have multiple effects[1-2]. Developmental defect is a problem, so that in United State 3-5% of fetuses have congenital anomalies. Approximately 2-3% of congenitad anomalies are related to teratogenic factor[3].Among the teratogenic factors considered are antiepileptic drugs (AED)[4]. Epilepsy is a chronic neurological disease that affects millions of people around the world and these people are forced to use at least one antiepileptic drug. Phenytoin is the most widely used in Iran by these patients[5]. Phenytoin C12H12N2O3 with the trade nameDiphenylhydantoin, is one of the oldest drugs for partial and tonic, generalized clonic seizure. Also phenytoin is used in treatment of cardiac arrhythmias three germinal neuralgia, ectopic secretion of anti-diuretic hormone, insulin and it is also used locally for wound healting.Therefore, it increases the number of people that use this drug[6]. Studies show that phenytoin exert significant effects on several physiological systems. It changes transport of sodium, potassium and calcium ions, membrane potential, concentraction of amino acids and neurotransmitters (norepinephrine, acetylcholine and GABA). Phenytoin absorption from the gastrointestinal system is nearly complete. Chronic administration of phenytoin may interfere with the metabolism of vitamin D and it causes osteomalacia[7-9].Another issue that shown in animal studies is that metabolites of phenytoin during pregnancy can cross from the blood-placental barrier easily and it can affect fetal organogenesis[10].Despite numerous studies, the teratogenic doses and specific days of antiepileptic drugs and their chemical components are still problems in the medical community and gynecology.This study aimed to assess the teratogeric effects of phenytoin drug experimentally by intraperitoneally injection on mouse embryo during organogenesis to determine effects of phenytoin on the process of the organogenesis to determine effects of phenytoin in the process of ossification of bones of the upper and lower limbs of the fetus in mice in days and the doses of these drugs.
2. Material and Methods
- This study was carried out on mature female NMRI mice, aged 2.5 months (7-8 weeks) old , and weighing 32±2 grams. Dry food pellets and water were provided with animal house conditions maintained at 20-22ċ, 50-55% relative humidity and a 12h: 12h photoperiod. Three famales were caged with a male of the same stain over night. The presence of vaginal plaque on the following morning confirmed that mating had taken place and was designated as day 0.5 of pregnancy (GD 0.5). Forty pregnant mice entered the 3 experimetal groups (10 mice in each group) receiving 60 mg/kg/day phenytoin in experimental groupI, 75 mg/kg/day phenytoin in experimental group II, and 90 mg/kg/day phenytoin in experimental group III, and one control group that only recived normal saline. The injections were out interaperitoneally (IP) from GD6.5 to GD14.5 (from day 6.5 to day 14.5 of pregnancy) with volume of 0.2 cc and the control group received normal saline with the same volume on 7 oclock in the morning. On GD18, the preganat mice were sacrificed under chloroform anesthedia and the uterus was opened and fetuses were exited. Then two fetuses were selected per mouse randomly and after removing the skin, viscera were removed , fixed in 95% ethyl alcohol for 3 day and for 3 day in acetone to remove tissues fats. Then samples were placed in alizarin red (made from merck) Alisan blue (made from merck) in the incubator at 38-40℃ for 4 days.Next, the fetuses were placed in a KOH and glycerin solution[11-12] and skeletal system status of fetuses was studied with stereomicroscope equipped with a calibrated lens (micrometr) and the amount of ossification of the humerus, radius, ulna, tibia and fibula of experimental group and control group were measured. After that, the amount of the ossification of upper and lower extremities was photographed using Nikon D200 camera Data were analysed by Anova, T-test and Tukey using Spss software.
3. Results
- Statistical tests used in fetuses of the experimental group (I,II,III) and control group showed that the average of length of the humerus in the fetuses of the experimental groups (I,II,III) decreased significantly compared to the control group. Also, studies of average ossification of the radius (right, left) of the fetuses showed that, the radius of the right hand in the experimental groupIII decreased significantly compared to the control group.The results also showed that the length of ulna of the left hand in experimental group I, decreased significantly compared to the control group. Also statistical test used in average length of ossification at femur (right,left) of the fetuse showed that length of femur of fetuses in the experimental groups (I,II,III) decreased significantly compared to the control group.For tibia (right, left), it was detected that length of tibia (right, left) in the experimental group III and length of tibia of the left foot in the experimental group I decreased significantly compared to the control group.For fibula also (right, left), the length of fibula in experimental group (I.II,III) decreased significantly compared to the control group.
|
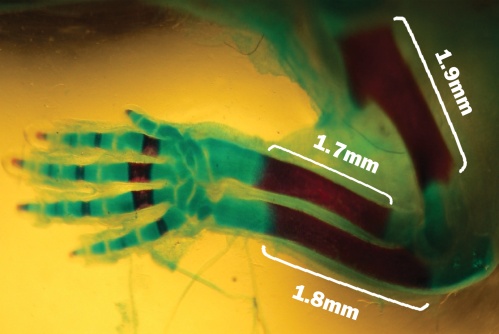
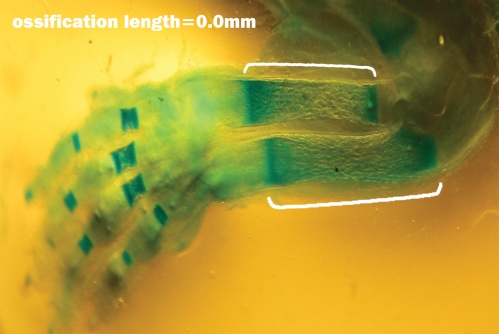 | Figure 2. Fetuse no ossification at Humerus bone, Radiuse bone and Ulna bone showed with Alizarin Red and Alcian Blue that related to the experimental group I |
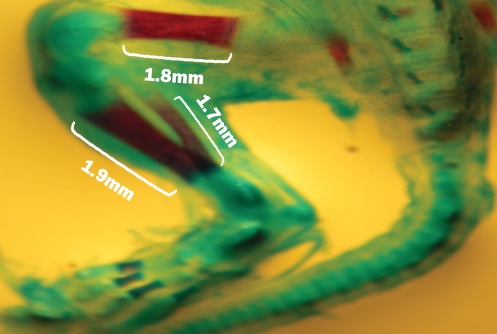
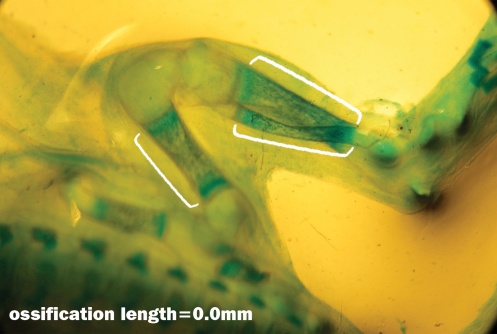 | Figure 4. Fetuse no ossification at Femur bone, Tibia bone and Fibula bone showed with Alizarin Red and Alsian Blue stained that related to the experimental group III |
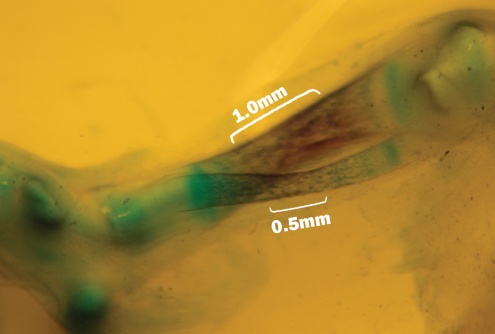 | Figure 5. Fetal with decreased ossification at Tibia bone and Fibula bone that showed with Alizarin Red and Alcian blue stained that related to the experimental group II |
4. Discussion
- The most important concern in production of a drug is its potential determine the teratogenic effects on human health. The first priority in indentifying whether a substance has teratogenic effects or not is testing on animals. This study was related to the effects of phenytoin on ossification during the organogenic on fetuses of mice. This study examined the effect of phenytoin on ossification on mice fetuse during organogenesis. This study showed that the incidence of teratogenic effects of this drug is associated to dose and days that it has been used in pregnancy. According to the study, the use of phenytoin during pregnancy can be considered as a risk factor for the development of the fetus and during organogenesis, it can reduce ossification of upper and lower organs of mice fetuses.In this study, in fetuses of the experimental group (I,II,III) and control group showed that the average of length of the ossification in the fetuses of the experimental groups (I,II,III) decreased significantly compared to the control group.Previous studies have shown that increasing the dose is increased teratogenic effects.But in this study show that increase of teratogenic independent of the dose. So that in experimental group II, the lowest teratogenic effects seen. This mechanism causes this result in experimental group II isn`t known so that should be study in molecular studies.Phenytoin has important shortcomings, such as non-linear and highly variable pharmacokinetic property and it has a suboptimal reaction rate. The main mechanism of phenytoin is to adjust and stop the continuous attacks of neurons to convulsions by direct inhibition and blockage ofvoltage-gated sodium channels in nerve cell membranes and delay cells activity. It is likely that this drug alters secondary messenger activities such as CAMP and CGMP that can induce a variety of abnormalities[11-14]. Studies show that 90 to 95% of phenytoin binds to plasma protein then is spread quickly through the blood to tissue and liver and metabolized in the liver by enzymes of the cytochrome CYP2C19 and p450 CY2C9 on which the drug metaboliziation depends[15-16].Studies show that phenytoin use during implantation and early organogenesis can cause severe abnormalities on the ossification. Chang and Ahn studied effects of phenytoin and Phenobarbital in 1994 and found that prescribed period of drug is important in its effect on mineral density, so that in children who received the drug in the leng-term, mineral density of bone was changed significantly[17]. Also studies show that skeletal abnormalities in fetuses whose mother had received were receiving some of the antiepileptic drugs during pregnancy, including phenytoin, could be attributed to role of this drug in metabolism of calcium, vitamin D and alkaline phosphatase. Vitamin D is involved in the absorption of Ca and excretion of phosphate and its deficiency will lead to decreased calcium deposition during ossification[18-20]. The use of phenytoin during pregnancy and coincided to the organogenesis, mat have teratogenic potential on spinal canal of fetus and causes disruption of growth and skeletal development of fetuses.Studies show that in the early stages of embryonic development, HOX genes are responsible expression of other genes and finally will result in a particular area. But teratogenic agents can cause mutation and congential anomalies[21]. Studies show that teratogenic effects of antiepileptic drugs such as Phenytoin is altered HOX genes expression, also the genotypes of the parents maybe have influence[22].To further explore the effects and mechanism of its action, more studies are needed .Since previous studies refer to decreased folate and methionine, change in metabolism of calcium and vitamin D, extensive studies on the effects of prophylactic folicacid ,methionin,vitamin D, vitamin E in various doses one recommended to be conducted.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML