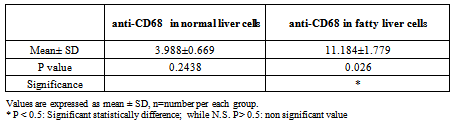-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2013; 3(6): 126-132
doi:10.5923/j.ajmms.20130306.02
Assessment of Hepatotoxicity of Obesity in Female Patients’ through Histopathological, Biochemical and Immunohistochemical Study
Ragia M. Hegazy1, 2, Waleed H. Almalki1, Eman Farouk2, 3, Hala F. M. Kamel3, 4
1College of Pharmacy, Umm Al-Qura University (UQU), Makkah, Saudi Arabia
2College of medicine, Benha University, Egypt
3College of medicine, Umm Al-Qura University (UQU), Makkah, Saudi Arabia
4College of medicine, Ain Shams University, Egypt
Correspondence to: Ragia M. Hegazy, College of Pharmacy, Umm Al-Qura University (UQU), Makkah, Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Background: Obesity is a multifacterial condition that involve complex interactions of metabolic, physiologic, social and behavioral factors. Obese individual has an increased incidence of non alcoholic fatty liver disease (NAFLD) which is one of the most common causes of chronic liver disease. The complications of NAFLD remain undescribed. Aim of the work: was to determine the progression of complications of NAFLD. Material and Methods: 20 liver biopsies from female patients’ were evaluated and categorized, according to the NAS(Non Alcoholic ActivityScore) system. Biopsies were processed for histopathological and immunohistochemical study beside liver function tests. Results:Liver biopsies from obese person showed single or multiple fat droplets inside the cytoplasm of the most of liver cell, ballooning of hepatocytes with positive immunohistochemical reaction for Mollary bodies occurred. Moreover inflammatory cells were detected in hepatic lobules together with hepatic cell necrosis. These changes were associated with pericellular ad peiportal fibrosis. There were significant increases in liver functions (P< 0.05). Conclusions:It is likely that regular assessment of liver functions and histopathological changes in obese will continue to play an important role in the diagnosis and management of people with NAFLD. Recommendations:Further studies are still required to determine the role of liver biopsy and liver function tests in monitoring therapeutic responses. The grading and staging NAFLD also requires further evaluation.
Keywords: Obesity, Liver Functions, Immunohistochemistry, NAFLD, Liver Biopsy
Cite this paper: Ragia M. Hegazy, Waleed H. Almalki, Eman Farouk, Hala F. M. Kamel, Assessment of Hepatotoxicity of Obesity in Female Patients’ through Histopathological, Biochemical and Immunohistochemical Study, American Journal of Medicine and Medical Sciences, Vol. 3 No. 6, 2013, pp. 126-132. doi: 10.5923/j.ajmms.20130306.02.
Article Outline
1. Introduction
- Obesity has reached epidemic proportion with a prevalence that continues to increase;obesity is due to combined effects of multiple gene, lifestyle andenvironmental factors.There is significant paralleling rise in incidence of obesity and diabetes[1, 2]. Non alcoholic fatty liver diseases (NAFLD) emerging as one of the most common cause of chronic liver disease[2, 3].NAFLD occurs in the setting of metabolic syndrome in which insulin resistance plays key role.Metabolic syndrome is a metabolic disorder related to obesity[4]. Obesity, increases delivery of free fatty acid to the liver,fatty acid metabolism is impaired with accumulation of triglyceride in liver of obese persons suggesting a defect in mitochondrial lipid oxidative accumulation of tissue triglyceride and acylCOAenzyme derivative may activate serine kinase pathway that induce insulin resistance by blocking the insulin receptors leading to type 2 diabetes mellitus[4, 5].Biochemical and Histopathological assessment will continue to play an important role in the diagnosis and management of people withNAFLD[6-8]. Liver biopsy is appropriate methods to determine the severity of liver damage accurately and may reveal an additional cause of liver damage[3, 9, and 10]. Causes of NAFLD remain un-described so the aim of the present study is to determine the complications of NAFLD through analysis of the changes in liver histopathlogy and its relation with liver function tests deterioration.
2. Materials and Methods
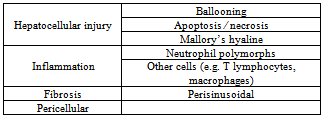
- SubjectsTwenty females were involved in the study had been subdivided into two groups. Group (1): Five female patients with normal body mass index ≤ 25 were used as control. Group (2): fifteen female obese patients[body mass index (BMI) ≥ 35 were enrolled prospectively and consecutively in the study. All patients underwent bariatric surgery at the Benha University Hospital.Exclusion Criteria: werepatients being treated with hepatotoxic or antineoplastic drugs, or with primary liver disorders other than fatty liver that could account for HcS. Ethical approval: An ethical approval for this study from Ethics Committee in Benha University Faculty of MedicineHospitals and all subjects provided written informed consent about the aim of the study had obtained before conduction of the study. Biochemical assayThe serum was extracted from each blood samplefor spectrophotometric determination of the levels of γ - glutamyltransferase (GGT) , aspartate transaminase(AST) and alanine transaminase (ALT)[11], alkalinephosphatase (ALP)[12], total protein[13], and albumin[14]. Also, the globulin valuesand albumin/globulin ratios were calculated.Histopathological study:Liver biopsies were obtained at the moment of bariatric surgery, using a Tru-cut of 18-G. Only samples longer than 14mm on histological examination were included in the study[20,21]. Liver biopsies were obtained from obese patients. Liver biopsies were immediately fixed in buffered formalin and processed for paraffin section ; Sections were obtained and stained with hematoxylin and eosin, ,to assess cumulativehepatocyte changes and necroinflammatory, fibrotic orarchitectural modifications and Masson trichrome stain to detect fibrosis. The intensity, distribution (centrizonal, panzonal and azonal) and quality (macrovesicular , multivesicular or mixed) of the HcS were analyzed. The term multivesicularHcS is used to refer to hepatocytes showing multiple small-medium sized vesicles without nuclear displacement to the cell periphery. This was assessed as present orabsent. In addition, we noted the presence or absence of lobular inflammation, pericellular and portal fibrosis and of any other hepatocellular injury. So features of steatohepatitis include hepatocellularinjury (beyond simple fatty change), inflammation and fibrosis (Table 1). Hepatocyte ballooning is the most characteristic feature of steatohepatitis[16].
|
3. Results
3.1. Biochemical Results
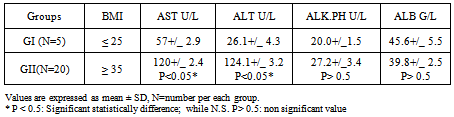
- Serum activities of transaminases, SGPT, SGOT and ALP, and serum albumin are given in Table.1 where the single dose of APAP significantly elevated SGPT, SGOT activities when compared to normal animals (Table.1).Subjects involved in this study were twenty five female patients who subdivided into two groups according to their body mass index; where Group 1: BMI≤ 25; while Group 2 BMI ≥ 35.
|
|
|
3.2. Morphometric Results
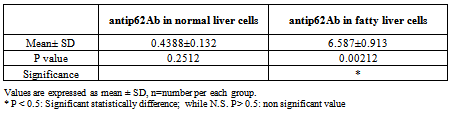
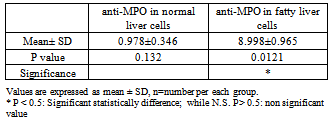
- The area % of positive anti-CD68 immunostaining, antip62Ab immunostaining and anti-MPO –myeloperoxidase - Abimmunostaining cells in liver significantly increased in obese persons as compared to control group ( table 1,2,3, histogram. 1,2,3).
|
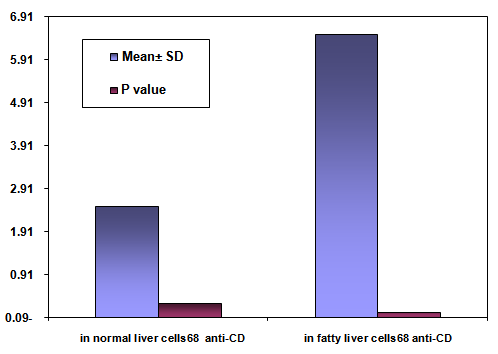 | Histogram (1). Showing the mean area % of anti-CD68 immunostaining, SD and P value in normal liver cells vs. fatty liver cells |
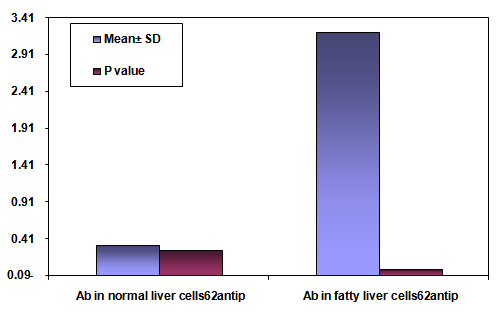 | Histogram (2). Showing the mean area % of antip62Ab immunostaining SD and P valuein normal liver cells vs. fatty liver cells |
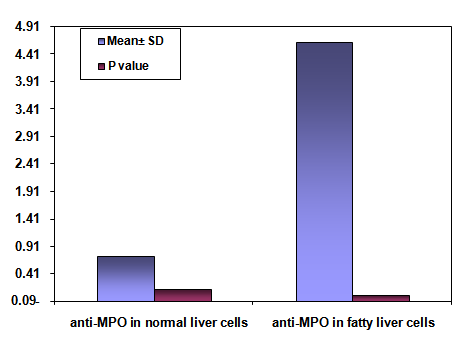 | Histogram (3). Showing the mean area % of anti-MPO -myeloperoxidase- Abimmunostaining SD and P valuein normal liver cells vs. fatty liver cells |
3.3. Histological Results
- The liver of the control group showed hepatic lobules. The center of the lobule was the central vein(Fig, 1). hepatic cords separated by sinusoids and showed minimal amount of collagen fibers around central vein(Fig, 2).
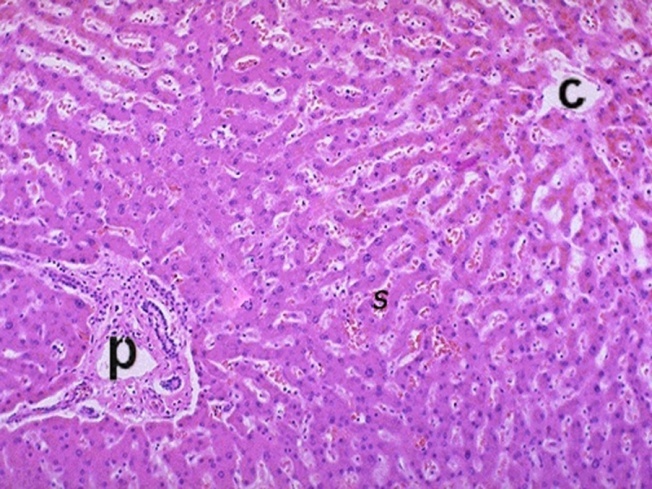 | Figure (1). Aphotomicrograph of a section ofcontrol liver,showing central vein (c). Radiating cords of liver cells surrounded by sinusoids and portal area (p). (H&Ex200) |
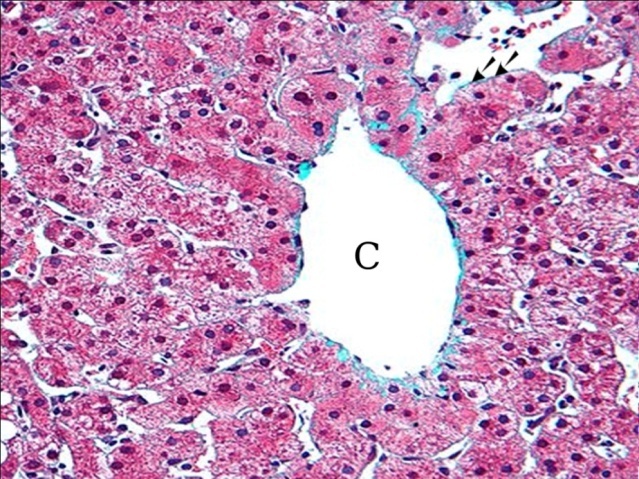 | Figure (2). A photomicrograph of a section of control liver, showing minimal amount of fibrotic tissue around central vein (c). (Masson trichrome x400) |
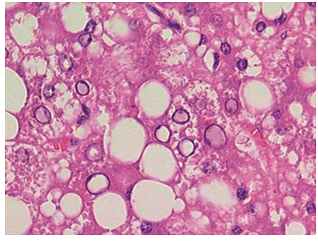 | Figure (4). A photomicrograph of a section of liver from obese person, showing some hepatocytes with glycogenated nucleus (arrow) (H&Ex400) |
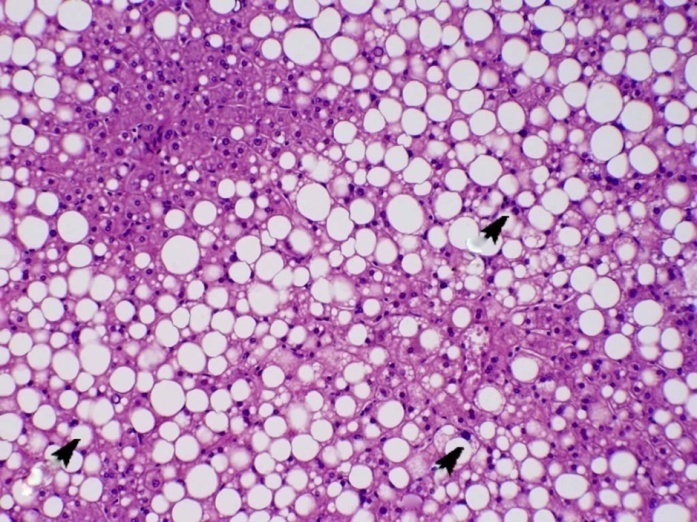
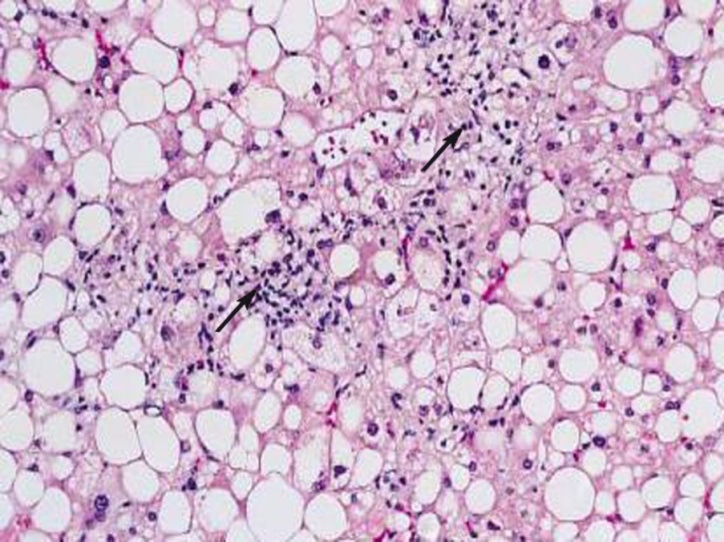 | Figure (6). A photomicrograph of a section of liver from obese person, showing fatty liver with cellular infiltration (arrow) (H&Ex400) |
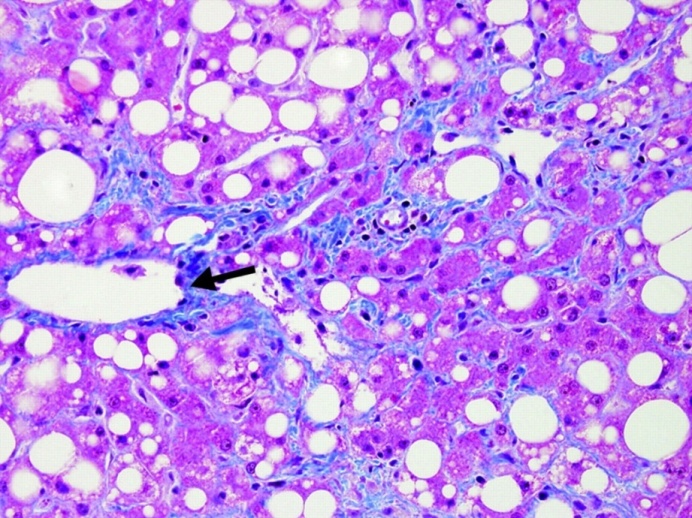 | Figure (7). A photomicrograph of a section of liver from obese person, showing fatty liver with perivascular fibrosis (arrow). (Masson trichrome x400) |
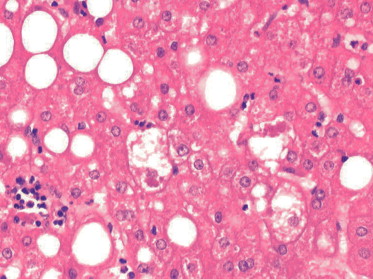
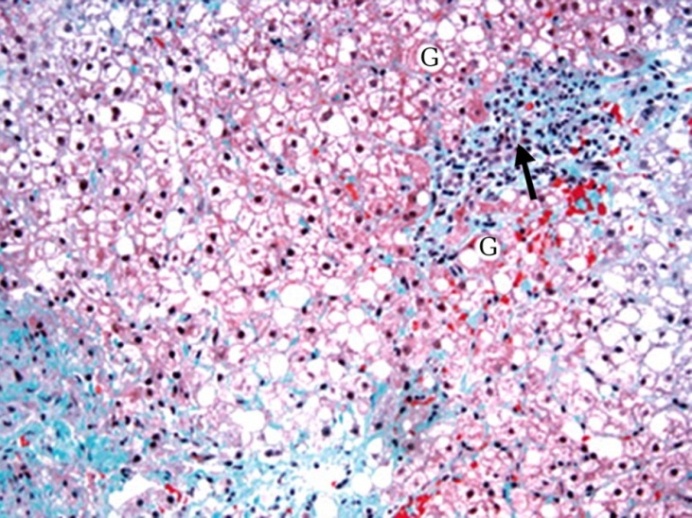 | Figure (8). A photomicrograph of a section of liver from obese person, showing hepatic cell necrosis (G),cellular infiltration and fibrosis (arrow).(Masson trichrome x400) |
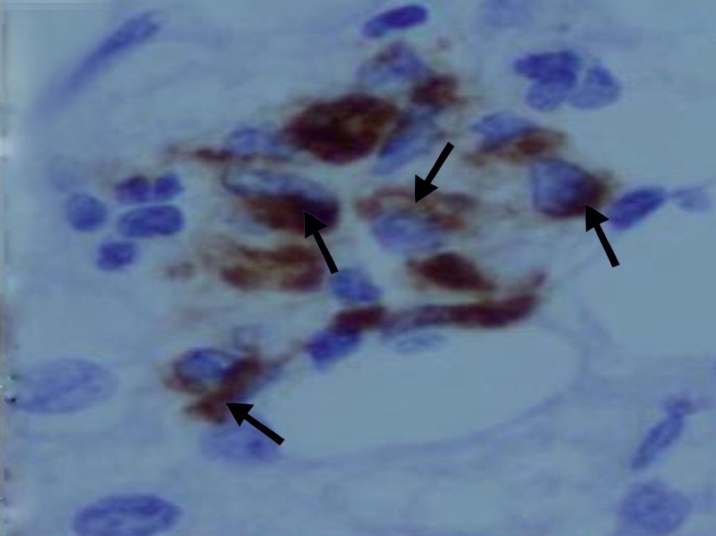
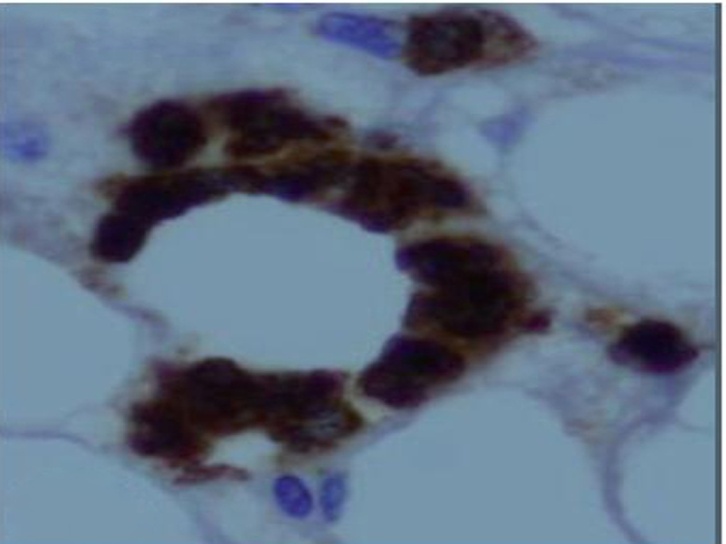
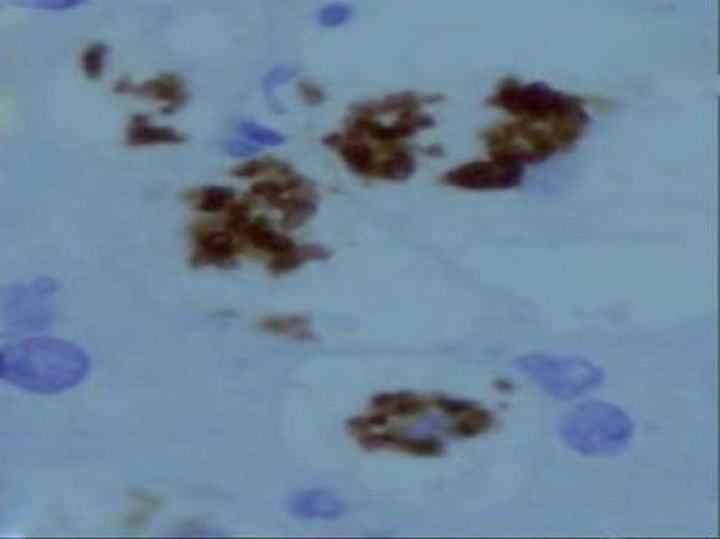 | Figure (9). A photomicrograph of a section of liver from obese person, showing macrophages aggregation in necrotic areas. (anti-CD68 Abimmunostaining x 400) |
4. Discussion
- Fatty liver is a metabolic syndrome related to increases delivery of free fatty acid to the liver of obese individuals[5,6]. The present study showed that the cytoplasm of a great number of hepatocytes were distended with single large fat droplet that displaced the nucleus to the periphery (macrovesicularsteatosis). Some hepatocytes showed microvesicularsteatosisin the form of small multiple vesicles. These results were in accordance with other investigators[10, 17]. Moreover, hepatocellular injury in the form of ballooning and necrosis was recorded in the present work. Also, some authorsdeclared that when the capacity of liver cells to store fat was decreased, fatty acid become toxic to the cells leading to cell death[13, 17]. Lipid mediators secreted by adipocytes like tumor necrosis factor and other metabolites could lead to liver cell death[10].Fatty liver was associated with increase expression of the membrane receptors rendering the cells susceptible to apoptosis[18-20]. In the present study, Mallory bodies were irregular acidophilic material composed of intermediated filaments aggregates. These bodies were associated with steatohepatitis. Inflammatory cells neutrophils and macrophages were detected in the liver parenchyma of the present work. Mitochondrial dysfunction associated with NAFLD might lead to release of reactive onto species.This lead to release of proinflammatory cytokines[21]. Moreover inflammatory mediators was released from adipose tissue leading to inflammatory changes[21-23].Hepatocytes with vacuolated (glycogenated nucleus )were seen in the present work. Some scientist declared that the presence of these nuclei denoted glycogen accumulation occurring in the setting of insulin resistance[4].Pericellular and periportal fibrosis were detected in the present study. Also some investigators declared that fibrosis was due to mitochondrial injury or due to mobilization of profibrogenic cytokines in response to hyperglycemia and inflammatory necrotic change[10, 20, and 21]. Progression of liver fibrosis was dependent on the presence of diabetes[4,14, and 15]. Other work, declared that fibrosis was recognized as indicator of liver damage and was the best prognostic markers for morbidity and mortality[24-26]. The present study revealed aggregation of Kuffers cells and stimulation to phagocytic debris and release of interleukinI (IL I) and tumor necrosis factor (TNF).Meanwhile, the present work showed extensive periportal fibrosis with apparent reduction in the severity of steatosis and steatohepatitis.
5. Recommendations
- Sustained weight reduction in association with exercise can decrease fatty liver, fatty inflammation and fibrosis and reversed insulin resistance and not need for medical treatment.Regular assessment of liver function tests beside liver biopsies ofobese adults, should be appliedto define the existence of NAS or otherwise of NASH.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML