-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2013; 3(5): 103-107
doi:10.5923/j.ajmms.20130305.02
Growth Inhibitory Effect of Senna siamea Leaf Extracts on Selected Microorganisms
Dahiru D.1, A. R. Malgwi1, H. S. Sambo2
1Department of Biochemistry, School of Pure and Applied Sciences, Modbbo Adama University of Technology, Yola, Adamawa State
2Department of Biochemistry, Faculty of Medical Sciences, University of Jos, Plateau State
Correspondence to: Dahiru D., Department of Biochemistry, School of Pure and Applied Sciences, Modbbo Adama University of Technology, Yola, Adamawa State.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Microorganisms are known to cause different types of diseases to both humans and animals. The use of medicinal plants to prevent and treat such diseases is increasing. This study evaluated the antimicrobial activity of different extracts (acetone, ethanol and water) and ethanol fractions (chloroform, ethyl acetate and n-butanol) of Senna siamea leaf on selected microorganisms, Escherichia coli,Staphylococcus aureus, Klebsiella pneumonia, Pseudomonas aeruginosa and Salmonella typhi using the agar well diffusion method. The extracts inhibited the growth of the microorganisms to varying proportions with zones of inhibition ranging from 2 to 18 mm. Water gave the highest yield of extract but with the least growth inhibition. Ethanol produced the highest zone of inhibition on Salmonella typhi, with the ethyl acetate fraction concentrating most of the phytochemicals with antimicrobial activity. The presence of alkaloids, saponins, tannins, glycosides, phenolics and flavonoids in the extracts could be responsible for the observed antimicrobial activity. The study confirms the use of Senna siamea leaf in the treatment of enteric fever in traditional medicine in different parts of the world.
Keywords: Senna siamea, Phytochemicals, Microorganisms, Zone of Inhibition
Cite this paper: Dahiru D., A. R. Malgwi, H. S. Sambo, Growth Inhibitory Effect of Senna siamea Leaf Extracts on Selected Microorganisms, American Journal of Medicine and Medical Sciences, Vol. 3 No. 5, 2013, pp. 103-107. doi: 10.5923/j.ajmms.20130305.02.
Article Outline
1. Introduction
- The application of herbs and medicinal plants in traditional medicine to diagnose, prevent or treat diseases dates back many centuries among rural communities throughout the world[8]. The active phytochemical principles produced by plants include, alkaloids, phenolic, anthraquinones, flavonoids, phenols, saponins, steroid, tannins, terpenes etc[15, 23]. In recent years, advances have been made in the development of antimicrobial compounds in an effort to check the harmful effects of microorganisms[4]. There is abundant number of medicinal plants and only small amounts of them are investigated for their biological and pharmacological activities. The wide range of medicinal plant parts like flowers, leaves, barks, stems, fruits, roots extracts are used as powerful raw drug possessing a variety of pharmacological activities[22]. Bacterial disease results when the harmful bacteria enter the organism then multiply and invade the body’s defence mechanism. These pathogenic bacteria enter the body through inhalation, ingestion or damaged skin tissue. The inability of the immune system to stop the bacteria from reproducing and spreading consequently results in the symptoms of bacterial disease[29]. Antimicrobial drug resistance is the foremost problem all over the world with present antibiotic therapy in treating infectious diseases[20].Senna siamea Lam. (Irwim and Barneby, Cassia siamea Lam) belongs to the sub-family Fabaceae (Caesalpinioideae) of the family Leguminosae[14]. Young leaves of the plant have been used as vegetables in Thailand[26] and as an antimalarial[25]. The stem bark extract was reported to have analgesic and anti-inflammatory effects[24]. Isolated compounds, Emodu and lupeol from the ethyl acetate fraction of the stem bark of Senna siamea were reported to be the active principles responsible for the antiplasmodial property with IC (50) values of 5µg/ml respectively[3]. Sub-chronic studies of the aqueous stem bark extract of the plant in rats did not show significant toxic effect after seven weeks of administration[21]. Escherichia coli is the most prevalent facultative gram negative rod in faeces, widely distributed in nature, and a frequent cause of infections of the urogenital tract, neonatal meningitis and diarrhoea. Escherichia coli cause diarrhoea due to enterotoxin[6]. Staphylococcus aureus (Gram positive cocci) is known to cause diseases like bacteraemia, pneumonia, osteomylitis, acute endocaditis etc[16]. Klebsiella pneumoniae (Gram-negative bacteria) mostly found in the tropics is associated with various diseases such as pneumonia, bloodstream infections and meningitis[18]. Pseudomonas aeruginosa is a gram-negative rod bacterium. It is a frequent cause of nosocomial infections such as pneumonia, urinary tract infections, and bacteraemia. Salmonella typhi is a genus in the family enterobacterium. It causes various human diseases such as gastroenteritis, bacteraemia and enteric fever[17]. Salmonellosis and enteric fever are always a public health concern in most developing countries, which are mostly low or middle-income countries with inadequate sanitation and hygiene, particularly regarding food, water, and disposal of human excreta[19] The use of Senna siamea leaf extract in the prevention and treatment of various diseases associated with some microorganisms prompted us to study the scientific basis for the use of the plant in traditional medicine. This was done by determining the antimicrobial activity of different extracts and fractions of Senna siamea leaf on selected microorganisms.
2. Materials and Methods
2.1. Plant Material
- Fresh leaves of S. siamae Lam. were collected within the University campus and authenticated at the department of Plant Sciences, School of Pure and Applied Sciences. The fresh leaves were shade-dried to constant weight for 7 days. The plant sample was crushed into smaller pieces using laboratory mortar and pestle and further reduced to powder using an electric blender. The powdered material was then stored in closed containers in a cool, dry and dark place until use.
2.2. Preparation of Aqueous and Organic Extracts
- The powdered sample (25 g) was placed in 500 ml conical flask and 200 ml of distilled water, acetone or ethanol was added to it separately. The suspension was allowed to sediment for 24 h with intermittent agitation of the flask. The extracts were obtained by filtering the suspensions using a clean sterile muslin cloth and then using Whatman filter paper (No. 1). The filtrates were then concentrated in vacuum at 40ºC and stored at 4ºC for further use[2].
2.3. Preparation of Fractions from Ethanol Extract
- The fractions from ethanol extract were obtained with chloroform, ethyl acetate and n-butanol as described by[13]. A 0.5 g of ethanol extract was mixed with 30 ml of chloroform in a separator funnel and allowed to soak for 5 min, and then shaken vigorously to mix. The chloroform soluble fraction (fraction A) was drained out into a sterile Petri dish and 30 ml of ethyl acetate was then added to the chloroform insoluble fraction, shaken properly and allowed to stand for 5 min. The ethyl acetate soluble fraction (fraction B) was also drained into a clean sterile Petri dish and 30 ml of n-butanol added to the insoluble fraction to obtain fractions C and D respectively. The soluble fractions were then evaporated to dryness and used for antibacterial susceptibility testing.
2.4. Phytochemical Screening
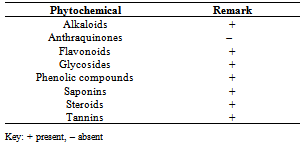
- Methods for the qualitative phytochemical screening were adapted from[31]. Ethanol extract was used for the phytochemical screening.Determination of tannins: About 0.5g of the extract was mixed with 10 ml distilled water shaken and filtered. To 5 ml of the filtrate was added 1 ml of 5% Ferric chloride solution. The appearance of blue black, greenish or blue green precipitate indicated the presence of tannins. Determination of flavonoids: A few drops of concentrated hydrochloric acid were added to a small amount of an alcoholic extract of the plant material. Immediate development of a red colour indicates the presence of flavonoids. Determination of anthraquinones: About 0.5 g of the extract was placed in a dry test tube and 5 ml of chloroform was added and shaken for 5 min using electric shaker. The content was filtered and equal volume of 100% ammonia solution was added and mixed together. A pink violet or red colour in the ammoniac layer indicated the presence of anthraquinones.Determination of saponins: About 0.1 g of powdered plant material was boiled with 10 ml of water for 5 minutes & filtered. After cooling 5 ml of filtrate was diluted with water & shaken vigorously. Determination of steroids: About 1 ml solution of extract was taken and then added to1 ml sulphuric acid. Red colour indicates the presence of steroid. Determination of alkaloids: About 0.5 g of the extract was stirred with 5 ml of 1% hydrochloric acid on a steam bath and filtered. About 1 ml of the filtrate was treated with few drops of Mayer’s reagent. White or creamy white precipitate considered as an indication for the presence of alkaloids. Test for phenolic compounds: About 1 ml of each of the concentrated extractives were heated to remove the solvent and the residues were taken in a little of aqueous methanol. To the methanol solution was added 0.5% ferric chloride solution and the change in colour was marked in alcoholic extract indicating the presence of phenolic compounds.Determination of Glycosides: A small amount of an alcoholic extract of the fresh or dried plant material was taken in 1 ml of water. Then, a few drops of aqueous sodium hydroxide were added. A yellow colour was considered as an indication for the presence of glycosides.
2.5. Test Organisms
- The microorganisms used included Escherichia coli, Staphylococcus aureus, Klebsiella pneumonia,Pseudomonas aeruginosa and Salmonella typhi. They were collected from the stock cultures of department of Microbiology, Modibbo Adama University of Technology, Yola. The isolates were characterized and maintained on nutrient agar slants at 4℃ prior to use.
2.6. Antimicrobial Assay
- The agar well diffusion method[1] was used. A 0.1 ml of diluted inoculums of test organism was spread on nutrient agar plates into the organ medium and filled with 200, 100, 50 and 25 mg/ml respectively with the plant extracts. The plate was incubated at 37℃ for 18 – 20 hrs. The antimicrobial activity was evaluated by measuring the zone of inhibition against each test microorganism.
2.7. Determination of Antibacterial Activity of the Fractions Obtained
- The dried fractions were each dissolved in methanol (2 ml) to get a final concentration of 200, 100, and 50 mg/ml. The solutions were impregnated on filter paper discs dried and used to determine their antibacterial activity.
2.8. Statistical Analysis
- Results are presented as means and simple percentages of three determinations.
|
|
|
3. Results
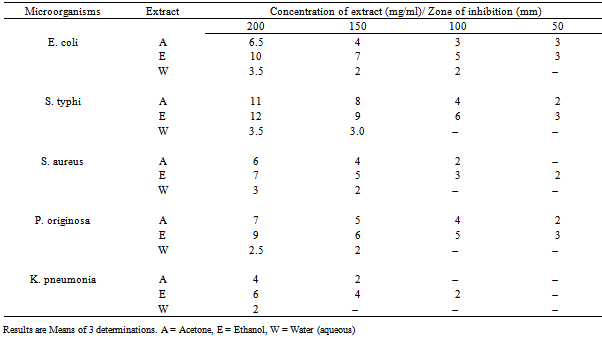
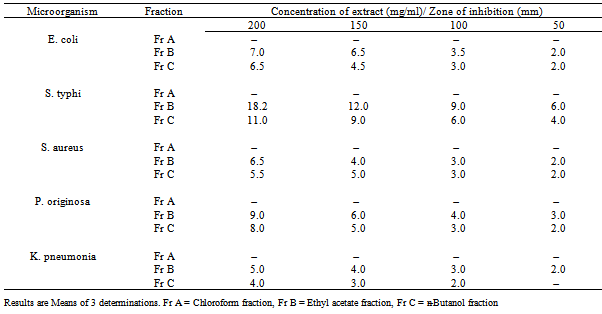
- The physicochemical characteristics revealed that aqueous extract gave the highest percentage yield (16.40 ± 0.6) followed by acetone extract (15.6 ± 0.3). All the three extracts obtained were dark green in colour, with the aqueous extract having the highest pH value of 4 while, acetone and ethanol extracts had a pH value of 3.8 each. All the extracts were acid in nature. Phytochemical screening of the aqueous extract of Senna siamea revealed the presence of alkaloids, tannins, saponins, glycosides, steroids phenols and flavonoids (Table 1).Antimicrobial activity of the three extracts at the various concentrations used, revealed that acetone and ethanol extracts inhibited the growth of all the microorganisms at the various concentrations while the aqueous extract could only inhibit the growth of microorganisms at 200 and 150 mg/ml. Ethanol extract gave the highest zone of inhibition (12 mm) at 200 mg/ml on salmonella typhi followed by acetone extract (11 mm) at same concentration and on same microorganism (Table 2). The antimicrobial activities of chloroform, ethyl acetate and butanol fractions of aqueous extract of Senna siamea leaf on the microorganisms tested showed that ethyl acetate fraction (Fr B) at 200 mg/ml had the highest zone of inhibition (18 mm) on Salmonella typhi (Table 3).
4. Discussion
- Extraction with water produced the highest percentage yield of extract though not significantly different from acetone extract, a strong indication that water was the best extracting medium. All the three extracts were acidic in nature and the phytochemical constituents may best function in acidic medium as reported earlier[11]. Presence of alkaloids, tannins, saponins, glycosides, steroids, phenolic compounds and flavonoids in all the extracts confirmed the presence of rich bioactive principles in the leaf. Tannins, steroids and glycosides had been reported in ethanol extract of the leaf of Senna siamea[5, 21] while alkaloids, saponins, phenolics and flavonoids by[22]. Secondary metabolites are mostly produced by plant during adverse condition for protection against herbivores[7]. Alkaloids, flavonoids, tannins and saponins were known to show medicinal activity as well as exhibiting physiological activity[12]. The presence of phenolic group in plants is to protect them from microbial, insect, and herbivores damage[8]. Many of these phenolic compounds also possess other functional attributes like antimicrobial, anti-inflammatory, antimutagenic, hypocholestemic and antiplatelet aggregation properties[27]. These phytochemical compounds carry out their activity by combining with protein, lipids or other components of the bacterial cell membrane that are relevant to one or more vital physiological roles thereby disrupting the integrity and functional behaviour of the membrane[10]. The highest zone of inhibition produced by the ethanol extract demonstrates that ethanol was a better extracting medium for the phytochemicals with antimicrobial activity. Salmonella typhi was the bacteria most inhibited by the extracts. Thus, several antibacterial agents inhibit the biochemical activities of microorganisms by distorting the structural arrangement of membrane phospholipids[4]. Growth inhibition ofantimicrobial agents does not only result from the loss of intracellular materials but instead may be dependent on the inhibition of fatty acid biosynthesis[30]. Ethyl acetate fraction of the ethanol extract had the best antimicrobial activity.
5. Conclusions
- This work has further confirmed the presence of bioactive principles in the extracts of Senna siamea leaf and the antimicrobial activity of the extracts. The highest zone of inhibition observed on Salmonella typhi justifies the use of the leaf in traditional medical practice for the treatment of enteric (typhoid) fever.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML