Jagtish Rajendran
Faculty of Medicine, Universitas Udayana, Denpasar, Bali, 80232, Indonesia
Correspondence to: Jagtish Rajendran, Faculty of Medicine, Universitas Udayana, Denpasar, Bali, 80232, Indonesia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Malaria is a serious and common problem among travellers all over the world especially travellers travelling to Indonesia. This descriptive study focus on artemisinin-based therapy for malaria especially in Indonesia. According to Centers for Disease Control (CDC), malaria is still present in most part of Indonesia. In April 2001, WHO recommended the use of artemisinin-based combination therapies (ACTs) to minimize the burden caused by Plasmodium falciparum resistance to conventional anti-malarial medications. Artemisinin is a sesquiterpene lactone produced from a plant called Artemisia annua. It is active against all Plasmodium species and its able to destroy all the blood stages of the parasite. Artemisinins causes less toxic effect if compared with other antimalarial agents. The year 2004 marks the beginning of adoption of artemisnin-based combination therapies (ACTs) into national malaria control program in Indonesia. 3 types of ACT which are dihydroartemisinin-piperaquine, artemether-lumefantrine andartesunate-amodiaquine. have been used recently to treat uncomplicated malaria in Indonesia. Resistance to artemisinin and its derivatives has been recently reported from Thailand-Cambodia border.
Keywords:
Malaria, Artemisinin, ACT in Indonesia, Resistance
Cite this paper: Jagtish Rajendran, Artemisinin-Based Therapy for Malaria, American Journal of Medicine and Medical Sciences, Vol. 3 No. 4, 2013, pp. 81-90. doi: 10.5923/j.ajmms.20130304.05.
1. Introduction
Malaria is transmitted by female Anopheles mosquito that usually bites from dusk to dawn which are infected by protozoan parasites species of Plasmodium genus. There are 4 types of Plasmodium which are Plasmodium falciparum, P. vivax, P. ovale and P. malariae. In addition, P. knowlesi, a parasite of Old World (Eastern Hemisphere) monkeys, has been identified in South East Asia[1,2]. Estimation by The World Health Organization (WHO) of reported cases from Indonesia were 2.5 million in 2006. Papua (300,000) and East Nusa Tenggara (70,000) provinces were the highest provinces of clinical malaria cases, annually[3]. Based on the 2011 World Malaria Report, in 2010, 4.3 million malaria cases were reported, of which 2.4 million wereparasitologically confirmed. Three countries accounted for 94% of confirmed cases: India(66%), Myanmar(18%) and Indonesia (10%). A total of 2426 malaria deaths were reported from eight countries, the great majority (93%) in India, Indonesia and Myanmar. Indonesia reported little change in the incidence of malaria between the year 2010 and 2011[4]. According to Centers for Disease Control (CDC), Malaria is still present in most part of Indonesia. Chloroquine-resistant P. falciparum malaria is present in Indonesia. Malaria is still endemic in all rural areas in Kalimantan, Sumatra, Sulawesi, and Nusa Tenggara Barat; areas of Bali outside the main resort areas; all areas of eastern Indonesia including Papua, Nusa Tenggara Timur, Maluku and Maluku Utara; and Lombok island. There is no malaria transmission in Jakarta; main resort areas of Bali or the island of Java (with the exception of Menorah Hills, central Java, risk still prevails there); and urban areas in Sumatra, Kalimantan, Sulawesi and Nusa Tenggara Barat. There is a low transmission in rural areas of Java[2]. In April 2001, WHO recommended the use of artemisinin-based combination therapies (ACTs) to minimize the burden caused by Plasmodium falciparum resistance to conventional anti-malarial medications such as chloroquine, sulfadoxine-pyrimethamine and amodiaquine. However, production and marketing of artemisinin monotherapies in the private sector of the endemic countries increases the chances of resistance to artemisnin. Due to this reason, WHO issued press release in January 2006 to stop the production of artemisinin monotherapies and was implemented in April 2006 at the WHO briefing on Malaria Treatment Guidelines and artemisinin monotherapies in Geneva. This step was taken to avoid development of resistance and compromise the effectiveness of ACTs[5].
2. Literature Review
2.1. Artemisinin
Artemisinin is a sesquiterpene lactone produced from a plant called Artemisia annua which also known as sweet wormwood, sweet Annie or "Qinghao". It is a chinese medicinal herb which has been used as treatment for fevers in China for more than 1500 years in the form of antipyretic tea. Artemisia annua is six-foot in height and has fernlike leaves. This plant grows in China and Vietnam and its origin believed to be from the Luofushan area of Guangdong which is a province in southern China. The active anti-malarial constituent qinghaosu was isolated from qinghao in 1971 by Chinese scientists and is named artemisinin. This plant is also now grown in Tanzania, South Africa, India and Madagascar[6-8].In Indonesia, at Tawangmangu, Central Java Province, the plant has been breeded since 2006 at a field taken care by the Health Ministry’s Agency for Research in Medicinal Plants and Traditional Medicines, after Indonesia got the seed from China[9].
2.2. Artemisnin Derivatives
Artemisinin is extracted and purified from the plant using solvent such as hexane and chromatography, respectively. The extract consists of artemisinin and its precursor, artemisinic acid which will be transformed into artemisinin. Artemisinin is a highly crystalline compound which does not dissolve in oil and water and can only be given by enteral route[6,7]. Organic reagents such as isoplugelol can be utilised for the total synthesis of artemisinin[8].Semi-synthetic derivatives of artemisinin was mainly formulated to overcome the poor bioavailability of artemisinin which reduce its effectiveness. Artemisinin undergo semi-synthetic process to producedihydroartemisinin then the process continue to produce a water soluble ester called artesunate and two oil soluble preparations called artemether and arteether (Figure 1)[6,7]. Artesunate is available in oral, rectal, intramuscular injection, or intravenous injection form. Artemether is available in oral, rectal or intramuscular form. Arteether is available in intramuscular injection form. Other derivatives of artemisinin are artelinic acid, artenimol and artemotil.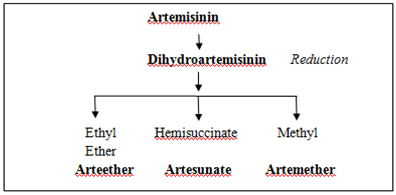 | Figure 1. Derivates of Artemisinin |
Chemical stability of these derivatives varies among one another. The least stable among these derivatives is dihydroartemisinin whereas artesunate is the most sensitive to humidity and to combination with amodiaquine. Due to acceleration of degradation by the presence of excipients, active substance powder mixtures are more stable than combination of powders in tablets. If artesunate and amodiaquine physically separated, they can only be used in a fixed dose combination. On the hand, a fixed combination of artemether-amodiaquine tablet does not demand this physical separation and is less vulnerable to high humidity situation[10].
2.3. Anti-Malarial Properties
The artemisinin derivatives are active against all Plasmodium species especially P. falciparum and P. vivax.. These derivatives are able to rapidly destroy all the blood stages of the parasite. These results in the shortest fever clearances times of all antimalarials. Artemisinin has blood schizonticides characteristics against P. falciparum and P. vivax. These drugs act on the blood forms of the parasite and thereby terminate clinical attacks of malaria[6].Artemisinin's main anti malarial properties comes from its endoperoxide bridge which can generate free radicals. Malaria parasites are very sensitive to unstable free radicals which are liberated through heme-catalyzed cleavage of peroxide. The plasmodium parasite ingests hemoglobin and releases free heme, an complex-moiety of iron-porphyrin during the infection of the red blood cells. Reactive oxygen radicals are produced from the reaction the complex and artemisinin. This causes the parasite to get damaged and eventually die. Plasmodium parasite requires conversion of toxic heme into non-toxic hemozoin, also known as malarial pigment, for its survival. Artemisinin inhibits the hemozoin formation activity of malaria parasite which ultimately leads to its destruction[6-8].Artemisinin and its derivatives also interfere with sarcoplasmic/endoplasmic calcium ATPase (SERCA), as well as damaging of normal mitochondrial functions of plasmodium parasites. Artemisinin works on the electron transport chain, yields local reactive oxygen species, and leads to the parasite’s mitochondrial membranedepolarization[6,8].Artemisinin is the fastest acting anti malarial available. It restricts the growth of the trophozoites and thus inhibits development of the disease. Circulating parasites are destroyed at early stage of life to avoid them from sequestering in the deep microvasculature. These drugs starts its action within 12 hours of consumption. These properties of the drug are very crucial in treating complicated P. falciparum malaria. These drugs are also active against the chloroquine resistant P. falciparum. strains.Artemisinin compounds have been reported to reduce gametocytogenesis, thus reducing transmission of malaria, this fact being specially significant in preventing the spread of resistant strains These drugs prevent the gametocyte development by their action on the ring stages and on the early (stage I-III) gametocytes which differ from other anti-malarial drugs available (Figure 2). It has broad stage specificity, destroying all asexual stages but not effective in exo-erythrocytic stages[7]. Due to this reason artemisinin is not recommended as prophylaxis for malaria infection.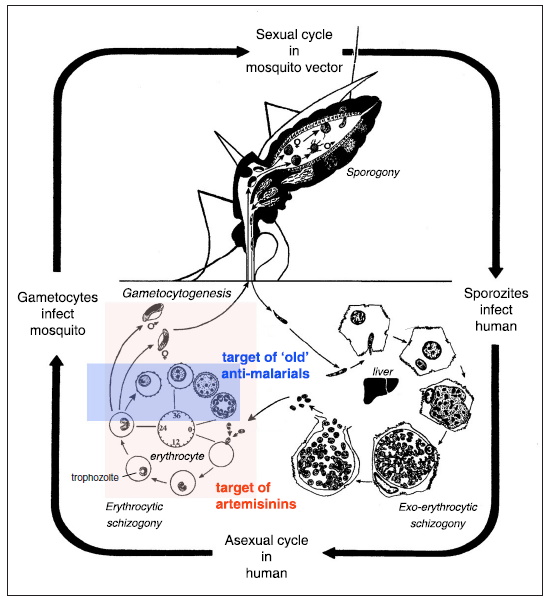 | Figure 2. Anti-malarial activity of artemisinin. Artemisinins kill parasites more effectively and at an earlier stage in the erythrocytic part of its life cycle than most of the other anti-malarial currently in use. They also kill the gametocyte stage and may contribute to interrupting transmission. They do not work on the exo-erythrocytic forms, hence do not prevent relapses in P. vivax or P. ovale (Hommel, 2008) |
2.4. Pharmacokinetics
Intra muscular or oral application of artemisininderivatives are well-absorbed. Oral formulations are generally quickly absorbed whereas intramuscular artemether has slow and erratic absorption. In treating severe malaria,intramusucular artesunate is pharmacokinetically superior to artemether for the treatment of, showing rapid and reliable absorption. Intrarectal artesunate shows rapid absorption and is a promising treatment for patients with moderate malaria when oral administration is not possible, and until hospital care is available[6].Most essential artemisinins are metabolised todihydroartemisinin in which form they have comparable antimalarial activity. Dihydroartemisinin has elimination half-life of about 45 min, It is rapidly cleared, predominantly through the bile[6]. Monotherapy treatment of these drugs is related with high incidences of recrudescent infection. Due to this reason it has been recommended to combine these drugs with other antimalarials to increase its efficacy.
2.5. Limitations of Artemisinins
Artemisinins causes less toxic effect if compared with other antimalarial agents. Artemisinin derivatives are well tolerated by patients[11]. The most common adverse side effects that have been identified are nausea, vomiting, anorexia, and dizziness; these are probably due, in many patients, to acute malaria rather than to the drugs[6,8].Neurotoxicity is the greatest concern regardingartemisinins. Intramuscular dosing was dicovered to be more toxic than oral dosing in many extensive studies conducted in many species and that, by any route, artemisinins which are fat-soluble were more toxic than artesunate. In addition, pharmacokinetic studies of oil based formulations of parenteral artemether and arteether have revealed the slow release and consequently long exposure times seen in both animals and humans. However, time of exposure to artemisinins influences neurotoxicity if compared to the maximum concentrations reached.Artemisinin is reported to affect areas in the brain stem such as the reticular system with regard to autonomic control, the vestibular system, the auditory system (trapezoid nucleus), and the red nucleus, which is important for coordination. Ataxia, slurred speech, and hearing loss have been reported in few patients treated with artemisinin.[11]. However artemisinins is still considered safe if compared to other anti -malarial drugs.
2.6. Alternative Production of Artemisinin
Alternative to full chemical synthesis of artemisinin was developed because the initial process is too complex and expensive. The alternative product is fully synthetic peroxides (trioxanes or trioxolanes) which is similar to artemisinin including the key endoperoxide bridge but their mode of action is different. One trioxalane (Arterolane) appear to be highly potent against P. falciparum in vitro but the drug prove to be too unstable in vivo. Research has been done to develop more stable molecules and a hybrid trioxane-aminoquinoline (trioxaquine) was discovered recently to be a fully synthetic anti-malarial peroxide[7].An alternative to chemical synthesis is microbial genetic engineering. Artemisinic acid can be grown in Escherichia coli and, even more successfully, in Saccharomyces cerevisiae (mevalonate pathway). Keasling and colleagues successfully transferred 10 A. annua genes into E. coli to create an almost ‘plant-like’ environment in the bacteria [7,8].
2.7. Uses Outside Malaria
Beside its anti -malarial properties, artemisinin and its derivatives is believed to be effective against parasitic diseases, such asschistosomiasis, and it was discovered recently that artemisinin could be useful in curing cancer[7]. There is some ongoing research about artemisinin's effectiveness to inhibit certain viruses, such as human cytomegalovirus and other members of the Herpesviridae family (HSV-1, EBV), hepatitis B virus, hepatitis C virus, and bovine viral diarrhea virus[11]. The complete antimicrobial potential of this product is yet to be discovered.
2.8. Artemisinin-Based Combination Therapy (ACTs)
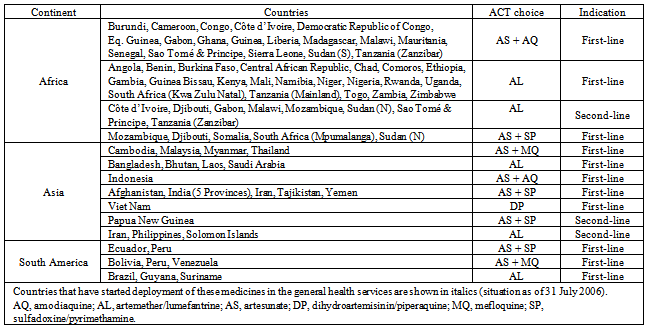
The evolvement of drug resistance especially toconventional malarial drugs such as chloroquine, sulfadoxine - pyrimethamin and amodiaquine, throughout the world is a serious challenge in combating malaria. In order to overcome this increasing burden, in April 2001, WHO recommended the use of artemisinin-based combination therapies (ACTs)[5].Global Fund to fight AIDS, Tuberculosis, and Malaria (GFATM) provides financial support for the major transition of adopting ACTs by malaria-endemic countries. US President’s Malaria Initiative (PMI) and the World Bank Booster Program, which were established in 2005 were additional resources to support this transition[12].However, production and marketing of artemisinin monotherapies in the private sector of the endemic countries increases the chances of resistance to artemisinin.Artemisinin monotherapy is prescribed for seven days to kill all parasites, but after a few days of consumption, patients usually stop the treatment when they begin to feel better. This causes the balance parasites to come in contact with low levels of the drug which maximize chances for resistance. Due to this reason, WHO issued press release in January 2006 to stop the production of artemisinin monotherapies and was implemented in April 2006 at the WHO briefing on Malaria Treatment Guidelines and artemisininmonotherapies in Geneva. This step was taken to avoid development of resistance and compromise the effectiveness of ACTs. 15 out of 41 participated manufacturers agreed to workhand-in-hand with WHO by stopping production and marketing of artemisinin as monotherapies[5,7].ACTs are recommended because artemisinin and its derivatives are fast acting but other drugs are often required to clear the body of all parasites and prevent recrudescence especially in treatment of uncomplicated malaria[8]. When artemisinins effectively kills most of the parasites in the first few hours of treatment, the partner drug comes in to action to kill the remaining parasites. This is attributed to different kinetic actions of the ACTs. Moreover, one drug protects the other drug from provoking resistance[7].The most common combinations of ACTs available are artemether-lumefantrine, artesunate-amodiaquine,artesunate-sulfadoxine-pyrimethamine, artesunate-mefloquine, and dihydroartemisinin-piperaquine. ACTs are more than 90% efficient in treating malaria[8]. Of the newer combinations, artemether-lumefantrine anddihydroartemisinin-piperaquine reported to be well tolerated with excellent efficacy in almost all the studies conducted worlwide[13].65 of 67 countries including 41 in Africa, implemented ACTs as their first-line treatment to treat uncomplicated falciparum malaria (Table 1). Only two countries adopted ACTs exclusively as second-line treatment. Among the ACTs, 28 countries adopted artemether/lumefantrin as first-line treatment, 19 countries opted for artesunate plus amodiaquine, artesunate plus sulfadoxine/pyrimethamine was chosen by 11 countries; artesunate plus mefloquine by 7 countries; and dihydroartemisinin/piperaquine by 1 country [12].By the year 2010, 84 countries already deployed ACT treatment in their public health sector. WHO always willing to provide continuous technical cooperation to ministries of health of all malaria-endemic countries on all aspects of national treatment policy change, monitoring therapeutic efficacy of medicines and implementing ACT-based treatment policies.
2.9. ACTs in Indonesia
The year 2004 marks the beginning of adoption of ACTs into national malaria control program in Indonesia. This adoption is due to the overwhelming burden of resistant malaria parasites to old conventional malaria drugs which affects more than 25% provinces in Indonesia. The Department of Health of Indonesia through discussion with malarial experts, Komisi Ahli Malaria (KOMLI) came to conclusion to adapt artemisinin-based combination therapies to treat malaria. This step was also in conjunction with WHO's recommendation of using ACTs to combat malaria[14,15].Table 1. Countries that have adopted ACTs and the ACT choices made by them (Bosman & Mendis, 2007)
 |
| |
|
2.9.1. Artesunate-amodiaquine
The first ACT adopted as first line treatment against malaria in Indonesia is artesunate-amodiaquine (Table 1). According to study conducted in 2009 by Asih et al. in West Sumba District, East Nusa Tenggara Province, it was concluded that artesunate-amodiaquine combination is an effective treatment for persons with uncomplicated P. falciparum malaria in this district which is one of the area with stable malaria infection[14].This non fixed dosed regimen is active against P. falciparum as well as P.vivax. The advantages of this ACT are safe for all ages, cheap and affordable whereas the disadvantage is poor compliance due to a lot number of pills need to be consumed. Moreover, in places such as Papua, Lampung, and North Sulawesi or in the areas with high chloroquine treatment failure, it was reported high treatment failure with artesunate-amodiaquine[15]. Consequently, the efficacy of artesunate-amodiaquine was varied (78–96%) [16].
2.9.2. Artemether-lumefantrine
Recently, artemether-lumefantrine is implemented in Indonesia. In the present study conducted by Sutanto et al., artemether-lumefantrine proved safe and highly efficacious in 59 residents of eastern Sumba Island presenting with uncomplicated falciparum malaria. Another study in southern Papua of Indonesia, an area with multidrug resistant P. falciparum also showed high cure rate (95.3%) of artemether-lumefantrine[17].In addition, a study in Papua demonstrated that there is less response to P.vivax compared to other combination (43%). When it is applied for malaria vivax, the regimen may be combined with doxycycline or tetracycline orclindamycin to increase sensitivity[15,16]. The disadvantage of this ACT is it should be administered twice daily for three days and given with fatty foods. Besides, it is expensive. All these data limits artemether-lumefantrine utilization as second line therapy to P. falciparum.
2.9.3. Dihydroartemisinin/Piperaquine
Dihydroartemisinin/piperaquine is next in line in treating malaria in Indonesia. The combination is chosen to overcome failure of the previous exixting combination such as artesunate-amodiaquine[15]. Based on the data of previous ACT trials in Indonesia, dihydroartemisinin / piperaquine is found to be the best ACT to treat uncomplicated malaria in areas with multi-drug resistance. In comparison with all the existing form of artemisinin, a 3 day dihydroartemisinin-piperaquine is the best substitute for the Indonesian ACT programme[16]. Besides, dihydroartemisinin/piperaquine is safe and effective for both P. falciparum and P. vivax malaria. The cure rates of DHP for the treatment of P. falciparum and P. vivax were reported 95.2% and 92.7%, respectively. It has been used for more than 2 years in Papua as the first line of therapy[16]. In addition, dihydroartemisinin/piperaquine had post treatment prophylactic effect against additional infection and relapse but study done by Arinaitwe et al. did not support the hypothesis that longer post-exposure prophylaxis should be associated with a reduction in the risk of additional infection in highly endemic areas. The cost of this ACT is similar to artesunate-amodiaquine[13].
2.9.4. Artemisinin-naphthoquine
This is the latest ACT which is being investigated in Indonesia because of its high efficacy in treating malaria. Naphthoquine which has longer half life (276 hours) initiate its antimalarial action by 72 hours and clear up any leftover Plasmodium parasites. Naphthoquine has good tolerance and it is safe. It is an anti-malarial substance discovered in the late 1980s by Chinese Academy of Military Medical Science. Although it has similarities in structure with choloroquin, it does not have any history of cross resistance[16]. In short, Artemisinin start the action, while Naphthoquine proceeds and maintain the course of the treatment.Based on limited Chinese clinical studies, a single dose administration of naphthoquine and artemisinin combination, was reported to be safe and effictive with cure percentage of 97.5% for treatment of P. falciparum malaria by day 28, and 90.0% for the treatment of P. vivax malaria by day 56. Tjitra et al. conducted a study in Jayapura and Maumere to compare the efficacy and safety of a single dose of artemisinin-naphthoquine with a three-day regimen of dihydroartemisinin-piperaquine, the existing programme drug, in adults with uncomplicated symptomatic malaria. Artemisinin-naphthoquine and dihydroartemisinin/piperaquine had day-42 polymerase chain reaction (PCR) corrected adequate clinical and parasitological response (ACPR) of ≥90% in intent-to-treat and modified population, and >95% in per-protocol population[16]. Data from this study is consistent with previous studies conducted to find the efficacy of AN for treatment of uncomplicated P. falciparum malaria in China Myanmar and Papua New Guinea. Based on this study, we can conclude that artemisinin-naphthoquine and dihydroartemisinin-piperaquine are confirmed very safe, effective, and tolerate for treatment of any malaria. Both of these drugs have the potential to be used for multiple first-line (MFT) therapy policies in Indonesia[16].
2.10. Treatment of Uncomplicated or Mild Malaria
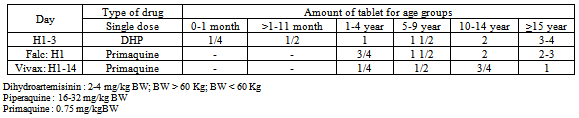
The treatment of choice for uncomplicated malaria is a combination of two or more anti-malarial medicines with different mechanisms of action. In Indonesia, 3 types of ACT have been used recently which are Dihydroartemisinin - Piperaquine, Artemether -Lumefantrine and Artesunate - Amodiaquine.The currently used first line drugs are dihydroartemisinin -piperaquine[15]. This is currently available as a fixed-dose combination with tablets containing 40 mg ofdihydroartemisinin and 320 mg of piperaquine. A target dose of 4 mg/kg/day dihydroartemisinin and 18 mg/kg/daypiperaquine once a day for 3 days, with a therapeutic dose range between 2–10 mg/kg/day dihydroartemisinin and 16–26 mg/kg/dose piperaquine (Table 2)[18].As a first line or drug for fail treatment is combination of artemether-lumefantrine[15]. This is currently available as a fixed-dose formulation with dispersible or standard tablets containing 20 mg of artemether and 120 mg of lumefantrine. A 6-dose regimen over a 3-day course is the recommended treatment. The dosing is formulated based on the number of tablets per dose according to pre-defined weight bands of the patients (Table 3)[18].Lumefantrine absorption is enhanced by co-administration with fat. It is crucial to inform the patients or caregivers to consume this ACT immediately after a having meal or drink containing at least 1.2 g fat – particularly on the second and third days of treatment[18]. Combination with doxycycline or tetracycline or clindamycin is necessary to increase sensitivity when treating malaria vivax.ACT used as the third alternative is artesunate – amodiaquine[15]. This third - alternative is presently obtainable as a fixed-dose formulation with tablets containing 25/67.5 mg, 50/135 mg or 100/270 mg of artesunate and amodiaquine. Separate scored tablets containing 50 mg of artesunate and 153 mg base of amodiaquine, respectively, are also available in the blister packs form.[18].Table 2. Dose of dihydroartemisinin-piperaquine treatment in malaria falciparum (Harijanto, 2010)
 |
| |
|
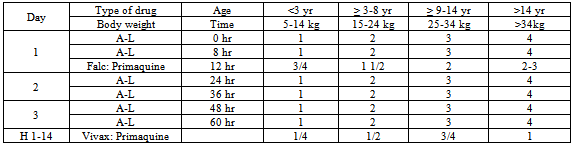
Table 3. Dose of artemether-lumefantrine (A-L) administration (Harijanto, 2010)
 |
| |
|
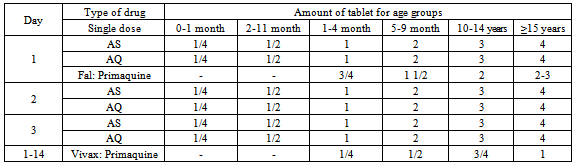
Table 4. Dose of artesunate-amodiaquine (AS-AQ) administration (Harijanto, 2010)
 |
| |
|
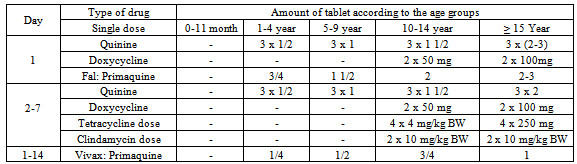
Table 5. Combination of Quinine + Doxycycline/Tetracycline/ Clindamycin (when the ACT fails) (Harijanto, 2010)
 |
| |
|
A target dose of 4 mg/kg/day artesunate and 10 mg/kg/day amodiaquine once a day for 3 days, with a therapeutic dose range between 2–10 mg/kg/day artesunate and 7.5–15 mg/kg/dose amodiaquine (Table 4)[18].When there is occurrence of ACTs treatment failure within 14 days of initial treatment, treatment can be continued with second-line anti-malarial drugs. WHO's recommended second-line treatments are, in order of preference:a) an alternative ACT known to be effective in the region,b) artesunate plus tetracycline or doxycycline or clindamycin (given for a total of 7 days),c) quinine plus tetracycline or doxycycline or clindamycin (given for a total of 7 days).One tablet of doxycycline 100 mg, dose 3-5 mg/kg BW once daily for 7 days, and tetracycline 250 mg or 500 mg, dose 4 mg/kg BW by 4 times a day are recommended. Pregnant women and children below 11 years of age are not allowed to use doxycycline/tetracycline, and it should be substituted with clindamycin 10 mg/kg BW, twice daily for 7 days. Table 5 describes the recommendation of the policy of the Indonesian Department of Health which indicates that when there is failure of ACT treatment, then the combination of quinine and tetracycline or clindamycin can be used. Primaquine should not be given to babies, pregnant women, and patients with G6PD deficiency[15].
2.11. Treatment of Severe or Complicated Malaria
Severe malaria is a medical emergency. Each patient suffering severe malaria should receive the following therapy such as general treatment, symptomatic treatment, administration of anti malaria drug and treatment for complication. Full doses of parenteral anti-malarial treatment should be started without delay with any effective anti-malarial first available.Parenteral drugs (intravenous or intramuscular) are preferred for anti-malaria drug treatment for severe malaria because in severe malaria, the drug should have capacity to eliminate parasite rapidly and remains long stay in blood system in order to rapidly reduce parasitemia stage[15].The largest ever real-life trial for severe malaria is SEAQUAMAT (Southeast Asia Quinine Artesunate Malarial Trial) of June 2003 to May 2005. 2,000 participants in Bangladesh, Myanmar and Indonesia took part in this trial. They were divided into two groups where one group was given quinine and the other group artemisinin. Due to high difference in mortality between the two groups, the study had to be stopped early. According to Arjen Dondorp of Mahidol University in Thailand, a leader of the study, “Artemisinin is better than quinine in all subgroups of patients with severe malaria. Artemisinin is the treatment of choice for severe malaria”[19].Artesunate injection is the first choice of treatment for severe malaria. Intravenous dose of 2.4 mg/kg BW/ is used at time of administration. It is given at 0, 12, 24 hour and continuously in every 24 hour until the patient is conscious (Figure 3). The dose of every single use is 2.4 mg/kg BW. When the patient is conscious, then it shall be substituted with oral artesunate, i.e. tablet of 2 mg/kg BW until the day 7 and after that, it is continued by parenteral use. To prevent recrudescence, it should be combined with doxycycline 2x100 mg/day for 7 days or clindamycin 2 x 10 mg/kg BW for pregnant women/children[15]. Artemether, or quinine, is an acceptable alternative if parenteral artesunate is not available. Dose of artemether is 3.2 mg/kg BW IM given on admission then 1.6 mg/kg body weight per day Dose of quinine is 20 mg salt/kg body weight on admission (IV infusion or divided IM injection), then 10 mg/kg body weight every 8 hours. Infusion rate should not exceed 5 mg salt/kg BW per hour[18].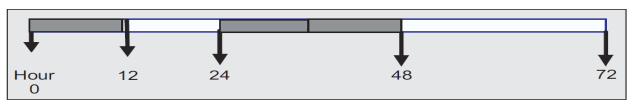 | Figure 3. Administration of artesunate (Harijanto, 2010) |
Table 6. Anti-malaria drugs for severe malaria (Harijanto, 2010)
 |
| |
|
2.12. Resistance to Artemisinins
The possibility of artemisinin's resistance to occur is very small due to the short half-lives of the drugs. However, increase usage of artemisinin, including monotherapy, has caused artemisinin resistance in certain part of the world[20]. Artesunate resistance have been reported lately from Cambodia. A study conducted in Pailin, western Cambodia found that P. falciparum parasites has decreased in vivo susceptibility to artesunate if compared with northwestern Thailand parasites. The resistance was distinguished by markedly increase time to clear the parasites. These decreased responses could not be explained by neither pharmacokinetic nor other factors[21].Some parasites discovered from French Guiana and Senegal showed decrease in vitro sensitivity to artemether. It has also been found that the effectiveness ofartemisinin-based combination agents has reduced alongThailand–Cambodia border. A ten year study (2001 to 2010) by 'Lancet' by researchers at the Shoklo Malaria Research Unit on the border of Thailand and Myanmar, measured the parasite clearance time from bloodstream. This research was participated by 3202 patients with falciparum malaria consuming oral artesunate-containing medications[22].The study found that over the ten years the parasite clearance time has increased from 2.6 hours in 2001 to 3.7 hours in 2010 which is a clear indication that the drugs were becoming less effective. The ratio of slow-clearing infections with half-lives of more than 6.2 hours has increased from 6 in 1000 to 200 in 1000. Later in the study, it was discovered that the decline parasite clearance rates was due to the changes in genetic of the parasites by examining the genetic make-up of the parasites. This caused the parasites to be resistant towards the drugs[22].In January 2009, WHO work hand-in-hand with Cambodia's and Thailand's health ministries to initiate a project to contain and eliminate artemisinin resistance from the border area. If this step is not taken, there is a possibility for the world to lose its best malaria treament. The WHO resistance containment activities consist of five steps which are[23].a) Stop the spread of resistant parasites.b) Increase monitoring and surveillance to evaluate the threat of artemisinin resistance.c) Improve access to diagnostics and rational treatment with ACTs.d) Invest in artemisinin resistance-related research.e) Motivate action and mobilize resources.Although it is tough to overcome artemisinin resistance issue, it is important in preventing artemisinin resistance from spreading to other regions in the world. All parties including scientific and clinical communities and health policy makers should work together to overcome this burdening issue. However, for the time being, the cases of true artemisinin resistance in malaria parasites is still low and this worry should not stop the usage of intravenous artesunate to treat severe malaria[20].
2.13. Challenges
According to data gathered by the Indonesian government, out of 1,322,451 suspected malaria cases recorded,ninety-two percent were examined by either microscope or rapid diagnostic test (RPD). Sixty-six percent out of 256,592 confirmed cases received ACT treatment.[24]Although there are evidences from previous studies claiming that ACT treatment are efficacious in combating malaria, the exact cure rates of malaria after implementation of ACT treatment in Indonesia is still not available. This is mainly due to the incoordination between health facilities such as public hospitals and private practices, and the Ministry of Health.According to 2012 World Malaria Report, the exact cure rates and trends of malaria in Indonesia is hard to be complied due to inconsistency of cases being reported to WHO.[25]Indonesian government which has strong treatment policy in fighting malaria, should take initiative to ensure the effectivity of ACT treatment remain intact and to ban distribution of counterfeit and substandard antimalarial drugs. These steps will make Indonesia to reach its goal to eliminate malaria by the year 2030.
3. Conclusions
Malaria is transmitted by female Anopheles mosquito which are infected by protozoan parasites species of Plasmodium genus. Estimation by The World Health Organization (WHO) of reported cases from Indonesia were 2.5 million in 2006. Indonesia reported little change in the incidence of malaria between the year 2010 and 2011. According to CDC, malaria is still present in most part of Indonesia. In April 2001, WHO recommended the use of ACTs to minimize the burden caused by P. falciparum resistance to conventional anti-malarial medications. Artemisinin is a sesquiterpene lactone produced from a plant called Artemisia annua which grows in China and Vietnam. Derivatives of artemisinin are artesunate, artemether, arteether, artelinic acid, artenimol and artemotil. Chemical stability of these derivatives varies among one another. artemisinin derivatives are active against all species of Plasmodium able to rapidly kill all the blood stages of the parasite. Artemisinin's anti malarial properties are generate free radicals, inhibits the hemozoin formation, interfere with sarcoplasmic/endoplasmic calcium ATPase (SERCA), as well as damaging of normal mitochondrial functions of plasmodium parasites. Most clinically important artemisinins are metabolised to dihydroartemisinin. Artemisinins causes less toxic effect if compared with other antimalarial agents. Artemisinin is believed to be effective against parasitic diseases and useful against cancer. ACTs are recommended because artemisinin and its derivatives are fast acting but other drugs are often required to clear the body of all parasites and prevent recrudescence. The year 2004 marks the beginning of adoption of ACTs into national malaria control program in Indonesia. In Indonesia, 3 types of ACT have been used recently to treat uncomplicated malaria which aredihydroartemisinin-piperaquine, artemether-lumefantrine and artesunate-amodiaquine. Artesunate injection is the first choice of treatment for severe malaria. Artemether, or quinine, is an acceptable substitute if parenteral artesunate is not available. Resistance to artemisinin and its derivatives has been recently reported from Thailand-Cambodia border. In January 2009, WHO work hand-in-hand with Cambodia's and Thailand's health ministries to initiate a project to contain and eliminate artemisinin resistance from the border area.
ACKNOWLEDGEMENTS
First of all, I would like to show my gratitude to the Lord because with his blessings I have managed to complete my paper entitled "Artemisinin-Based Therapy for Malaria". With pleasure, I, Jagtish Rajendran, would like to convey my heartiest thank you to dr. Ida Ayu Ika Wahyuniari, M. Kes, the supervisor for my paper work whose encouragement, guidance, and support from the initial to the final level enabled me to develop an understanding of the topic and successfully complete my paper work.I would like to apologize if there are any weaknesses that could be found in my report and I would be glad to get feedbacks from the readers as it would help me in improvising myself in time to come. Last but not least I hope that my writing would be beneficial to the readers as how it was for me.
References
| [1] | World Health Organization (WHO). World Malaria Report 2009. 2009. |
| [2] | Arguin PM and Mali S. Malaria, Chapter 3: Infectious Diseases Related To Travel, 2012 Yellow Book, Traveler’s Health, Centers for Disease Control And Prevention (CDC). Available from: http:// wwwnc. cdc. gov/travel/ yellowbook /2012/chapter–3–infectiou–diseases-related-to-travel/malaria.htm |
| [3] | World Health Organization (WHO). World Malaria Report 2008. 2008. |
| [4] | World Health Organization (WHO). World Malaria Report 2011. 2011. |
| [5] | World Health Organization (WHO). WHO briefing on Malaria Treatment Guidelines and artemisininmonotherapies. 2006. |
| [6] | Woodrow CJ, Haynes RK and Krishna S.. Artemisinins. Postgraduate Medical Journal. 2005;81:71-78. |
| [7] | Hommel M. 2008. The future of artemisinins: natural, synthetic or recombinant? Journal of Biology. 2008; 7(38): 38.1-38.5. |
| [8] | Obimba and Clarence K. The significance of artemisinin in roll back malaria partnership programmes and cancer therapy. African Journal of Biochemistry Research. 2012;6(2):20-26. |
| [9] | Gusmaini and Nurhayati H. Potensi Pengembangan Budidaya Artemisia annua L. di Indonesia. Perspektif. 2007;6(2):57-67. |
| [10] | Acker KV, Mommaerts M, Vanermen S, Meskens J, Heyden YV and Vercammen JP. Chemical stability of artemisinin derivatives. Malaria Journal. 2012; 11(S1):99. |
| [11] | Efferth T,1 Romero MR, Wolf DG, Stamminger T, Marin JJG, and Marschall M. The Antiviral Activities of Artemisinin and Artesunate. Clinical Infectious Diseases. 2008;47:804–811. |
| [12] | Bosman A and Mendis KN. A Major Transition in Malaria Treatment: The Adoption and Deployment of Artemisinin- Based Combination Therapies. The American Society of Tropical Medicine and Hygiene. 2007;77(S6): 193–197. |
| [13] | Price RN and Douglas NM. Artemisinin Combination Therapy for Malaria: Beyond Good Efficacy. Clinical Infectious Diseases. 2009;49:1638–1640. |
| [14] | Asih PBS, Dewi RM, Tuti S , Sadikin M , Sumarto W , Sinaga B, et al. Efficacy of Artemisinin-Based Combination Therapy for Treatment of Persons with Uncomplicated Plasmodium falciparum Malaria in West Sumba District, East Nusa Tenggara Province, Indonesia, and Genotypic Profiles of the Parasite. The American Society of Tropical Medicine and Hygiene. 2009;80(6): 914–918. |
| [15] | Harijanto PN. Malaria Treatment by Using Artemisinin in Indonesia. Acta Medica Indonesiana. 2010;42(1):51-56. |
| [16] | Tjitra E, Hasugian AR, Siswantoro H, Prasetyorini B, Ekowatiningsih R, Yusnita EA, et al. Efficacy and safety of artemisinin-naphthoquine versus dihydroartemisinin - piperaquine in adult patients with uncomplicated malaria: a multi-centre study in Indonesia. Malaria Journal. 2012; 11(153):1-14. |
| [17] | Sutanto I, Suprianto S, Widiaty A, Rukmiyati, Rűckert P, Bethmann AV, et al. Good Efficacy of Artemether - Lumefantrine for Uncomplicated Falciparum Malaria in Eastern Sumba, East Nusatenggara, Indonesia. Acta Medica Indonesiana. 2012;44(3): 187-192. |
| [18] | World Health Organization (WHO). Guidelines for the treatment of malaria: Second edition. 2010. |
| [19] | South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;36:717-725. |
| [20] | Rosenthal PJ. Artesunate for the Treatmentof Severe Falciparum Malaria. The New England Journal of Medicine. 2008; 358(17):1829-1836. |
| [21] | Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin Resistance in Plasmodium falciparum Malaria. The New England Journal of Medicine. 2009;361:455-467. |
| [22] | Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. |
| [23] | World Health Organization (WHO). Global Plan For Artemisinin Resistance Containment (GPARC). 2011. |
| [24] | Kusriastuti R and Surya A. New Treatment Policy of Malaria as a Part of Malaria Control Program in Indonesia. Acta Med Indones-Indones J Intern Med. 2012;44(3): 265-269. |
| [25] | World Health Organization (WHO). World Malaria Report 2012. 2012. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





