-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2013; 3(4): 74-80
doi:10.5923/j.ajmms.20130304.04
Toxicological Assessment of Ethanolic Extract of the Leaves of Newbouldia laevis (P. Beauv)
O. T. Kolawole 1, M. A. Akanji 2, M. O. Akiibinu
1Department of Pharmacology and Therapeutics, College of Health Sciences, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
2Department of Chemistry and Biochemistry, Caleb University, Lagos, Nigeria
Correspondence to: O. T. Kolawole , Department of Pharmacology and Therapeutics, College of Health Sciences, Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Newbouldia laevis (P. Beauv) is a medicinal plant traditionally used to treat various diseases across African countries. In Nigeria the leaves of the plant are used in the treatment of convulsions, diabetes, and different bacterial infections. In this study, the acute and sub-chronic toxicity profiles of the ethanolic extract of the leaves of N. laevis were evaluated. The effects of the extract on water intake and food consumption change in body weight, hematological and blood biochemical parameters like aminotransferases, creatinine and urea as well as histology of liver and kidney were assessed for morphological changes and inflammatory cells. The median lethal dose (LD50) of the extract was calculated to be 5400 mg/kg in albino mice. In the sub-chronic toxicity study, all the biochemical and hematological parameters assessed in the treated albino rats were not significantly different from the control except the platelet count that was significantly high at high doses. Likewise, examination of liver and kidney sections did not show any morphological changes and inflammatory cells infiltrations. The results of this study suggest that the ethanolic leaf extract of Newbouldia laevis has low toxicity profile regarding hematological, blood biochemical and histological parameters.
Keywords: Newbouldia laevis, Toxicity, Histopathology, Medicinal Plants
Cite this paper: O. T. Kolawole , M. A. Akanji , M. O. Akiibinu , Toxicological Assessment of Ethanolic Extract of the Leaves of Newbouldia laevis (P. Beauv), American Journal of Medicine and Medical Sciences, Vol. 3 No. 4, 2013, pp. 74-80. doi: 10.5923/j.ajmms.20130304.04.
Article Outline
1. Introduction
- One of the basic goals of researchers in their effort to discover new drugs is to develop new products with high therapeutic efficacy and low toxicity profile. To accomplish this, more attention has been given to medicinal plants in the recent years. This is because medicinal plants present a rich source of compounds that possess different therapeutic effects. However, most medicinal plants are yet to be thoroughly evaluated for their toxicity profiles. It is generally believed that medicinal plants and their products are safer than their synthetic equivalents. While in some instances this may be true, a blanket assumption that medicinal plants are free of toxic effects is not correct in its entirety. The fact that medicinal plants are of natural origin does not guarantee for their safety [1].Some medicinal plants that were once considered non-toxic have been reported to be hepatotoxic while some have been reported to be responsible for renal impairment [2, 3]. Therefore proper and detailed toxicological assessment should form a critical component of both early and late phases of drug development from medicinal plants to avoid toxicity tragedies after such drugs might have been approved for therapeutic purposes. One way to determine the toxicity profile of herbal preparations is to assess their effects on hematological and biochemical parameters [4]. Newbouldia laevis (P. Beauv) is a medicinal plant that belongs to Bignoniaceae family. It is native to tropical Africa and grows from Guinea Savannah to dense forests. It is found in Nigeria, Senegal, Cameroon, Gabon, Angola and some other African countries [5]. Its common names are ‘African Border Tree’ and ‘Fertility Tree’. In Nigeria, it is known by different indigenous names such as ‘Aduruku’ (Hausa), ‘Ogirisi’ (Igbo) and ‘Akoko’ (Yoruba). It is used by African traditional healers to treat various ailments like diabetes, rheumatism and toothache. In Nigeria, herbalists use decoction of the bark to treat epilepsy and convulsions in children. The leaves are soaked in ethanol for the treatment of diabetes and sickle cell disease. Different parts of the plant have also been reported to possess antimicrobial properties [6, 7]. In spite of the widespread use of the leaves of Newbouldia laevis as herbal remedy, reports on proper toxicological evaluation of the plant are lacking. In this study, we evaluated the acute and sub-chronic toxicity profiles of the ethanolic extract of the leaves of Newbouldia laevis using hematological, blood biochemical and histological parameters in albino mice and rats.
2. Methodology
2.1. Preparation of Plant Extract
- Leaves of Newbouldia laevis were collected from the premises of College of Health Sciences, Ladoke Akintola University of Technology, Mercyland, Osogbo Campus, Nigeria. The leaves were identified and authenticated by a taxonomist in Forest Research Institute of Nigeria (FRIN) and a voucher specimen was deposited in the herbarium of the institute (voucher specimen no: FHI 107753).The leaves were thoroughly washed with distilled water to remove soil and other debris that may contaminate the plant sample. The washed sample was then air-dried under shade in the laboratory for 5 days and the dry plant sample was pulverized using an electric grinding machine. The resultant powder sample weighing 500 g was then extracted with 80 % ethanol at 70℃ by continuous hot percolation using aSoxh- let apparatus. The extraction was carried out for 24 h and the resulting ethanolic extract was concentrated at 40℃ in a rotary evaporator. The solid sample obtained weighed 47.5 g (yield = 9.5%). The crude ethanolic extract was kept in air-tight container and stored in a refrigerator at 4℃ until the time of use.
2.2. Experimental Animals
- Albino mice of both sexes weighing 25- 30 g and Wistar rats of both sexes weighing 180- 200g were obtained from the Animal Holding Unit of the Department of Pharmacology and Therapeutics, Ladoke Akintola University ofTechnolo-gy (LAUTECH), Nigeria. All experimental procedures were conducted in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals [8] as well as Ethical Guidelines for the Use of Laboratory Animals in LAUTECH, Ogbomoso, Nigeria. The animals were housed in polypropylene cages inside a well-ventilated room. A maximum of six animals of the same sex were kept in one cage. The animals were maintained under standard labora-tory conditions of temperature (22 ± 2°C), relative humidity (55-65%) and 12 hour light/dark cycle. During the whole experimental period, animals were fed with a standard balanced commercial pellet diet (Ladokun Feeds Ltd. Ibadan, Nigeria) and potable tap water ad libitum. The animals were allowed to acclimatize for 2 weeks before the experiment.
2.3. Acute Toxicity Study
- Acute toxicity was studied in mice using the method of Lorke [9]. After overnight fasting, mice of either sex were randomly divided into 5 groups of 10 animals each. Five doses were chosen such that the smallest dose caused no death while the highest caused 100% mortality. Each of the five doses of the extract was administered by oral gavage to different group of mice. They were then allowed free access to food and clean water and observed for the first 2 hours and then at 6th, 12th and 24th hour for any toxic symptoms. The parameters observed were grooming, mood,hyperacti- vity, sedation, loss of righting reflex, respiratory rate and convulsions. After 24 hours, the number of mice that died was counted in each group and percentage of mortality was calculated. The percentage mortality at each dose level was then converted to probit. The probit values were plotted against log-doses and then the dose corresponding to probit 5 (i.e., 50%) was determined as the LD50 of the extract. Biostat 2007 5.1.4.0 (AnalystSoft) was used for theanalysis.
2.4. Sub-chronic Toxicity Study
2.4.1. Experimental Design
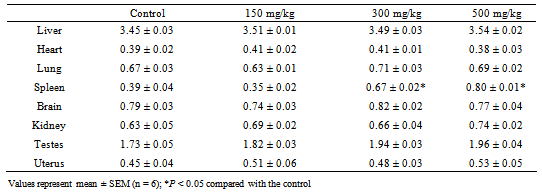
- Four groups (A, B, C, and D) of six rats each were used for the study. Groups B, C and D were, respectively,administered 150, 300 or 500 mg/kg body weight of the ethanolic extract orally by gastric gavage once daily for consecutive 28 days. The doses were chosen based on effective therapeutic doses of ethanolic extract of the leaves of N. laevis in rats as reported earlier [10]. Group A which served as the control was treated with normal saline (0.9% NaCl solution). Before the initiation of dosing, the rats were left for 7 days toaccli-matize to laboratory conditions. All animals were monitored for any deviations in normal behavior, fecal discharge, movements and mortality on a daily basis during the 28-day period of study. In addition, daily food intake of each group of rats was also monitored. The body weight (in gram) of each rat was recorded on day 0 and at weekly intervals throughout the course of the study and the average body weight for the groups was calculated. On day 28, the animals received the last treatment dose. Six hours later, each animal was anaesthetized with intraperitoneal injection of sodium pentobarbitone (40mg/kg body weight) and blood samples were collected by cardiac puncture into EDTAanticoagula- ted and non anti-coagulated tubes. The liver, kidney, heart, lung, spleen, brain, testes and uterus were excised off the body into separate bottles. The heparinized blood was used for hematological studies while the blood in nonanticoagu- lated tubes was centrifuged at 1500 rpm for 10 min and the sera collected into clean, dry tubes for biochemical analysis.
2.4.2. Food and Water Consumption
- The amount of food and water consumption was evaluated daily as known amount of food and water was given to each animal in each cage. After 24 h, the remaining food and water was taken from the cage and measured. To find out the food and water consumption, remaining amount of food and water was deducted from the total amount. Food intake was calculated as g/100g body weight/day. Water intake was calculated as ml/100g body weight/day.
2.4.3. Body Weight and Relative Organ Weight
- Body weight of the rats was measured every week for the four weeks of the experiment.Liver, kidney, heart, lung, spleen, brain, testes and uterus were collected, mopped with filter paper and weighed to determine the relative organ weight. The relative organwei-ght was calculated and expressed as g/100 g body weight using the following formula:

2.4.4. Hematological Analysis
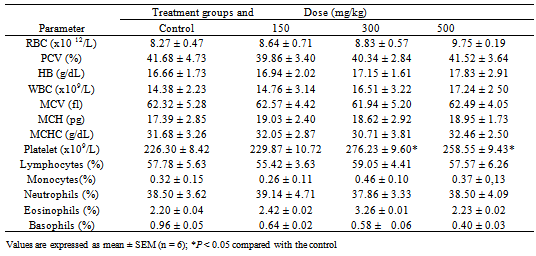
- Within ninety minutes after blood collection, the samples were subjected to hematological analysis on Sysmex XE-2100 (Sysmex Corporation, USA), a fully automated analyzer. The following hematological parameters were determined: red blood cell count (RBC), white blood cell count (WBC), hemoglobin (HB), packed cell volume (PCV), mean cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration(MCHC), platelet count, lymphocytes, monocytes, neutrophils, basophils and eosinophils.
2.4.5. Biochemical Analysis
- Serum aspartate aminotransferase (AST) and alanineamino transferase (ALT) activities were estimated by the method of Reitman and Frankel [11] using test kit (BioVision Inc. USA). Alkaline phosphatase activity was estimated by the method of Kind and King [12] using ALP test kit (BioVision Inc. USA). Serum creatinine was estimated by a commercial kit (Vitro Scient Co.) based on modified kinetic Jaffe reaction [13], while serum urea was estimated by a method based on modified Urease-Berthelot method [14] using commercial kit (Randox Laboratories Ltd, UK). Total bilirubin was estimated by an assay based on the method of Malloy and Evelyn [15]. Total protein and serum albumin were estimated by the method of Tietz,[16] and globulin was calculated by subtracting albumin from total protein value.
2.4.6. Histopathological Examination
- The method described by Aliyu et al. [17] was followed. After blood collection, the liver and kidney were carefully dissected from the abdominal region. They were preserved in buffered formalin for 72 hr and then sliced into a thickness of 2.5mm. The tissues were dehydrated with alcohol of graded concentrations. They were further embedded in paraffin wax and cast into blocks. Sections of the tissues were then cut on a microtome to 5µm. These sections were later spread onto a slide and allowed to dry. The slides were subsequently stained in hematoxylin- eosin and examined under a light microscope for morphological changes and infiltration of inflammatory cells. Photomicrographs of the samples were taken and interpreted.
2.5. Statistical Analysis
- Data obtained from the experiments are expressed as mean ± standard error of mean (SEM). The data were subjected to one-way analysis of variance (ANOVA) and Student’s - Newman-Keul test to determine the statistical significance of differences between groups. Differences were considered to be significant when P < 0.05. GraphPad Prism version 5.0 for windows was used for these statistical analyses (GraphPad software, San Diego California USA).
3. Results
- With the administration of up to 1000 mg/kg of the extract, mortality was not recorded and no adverse effects were observed in the animals. When the dose was further increased, behavioural changes such as reduced motor activity, sedation and unresponsiveness to auditory stimuli and light touch were observed. Mortality was also recorded and LD50 was calculated to be 5400 mg/kg from the dose-response curve (Figure 1).
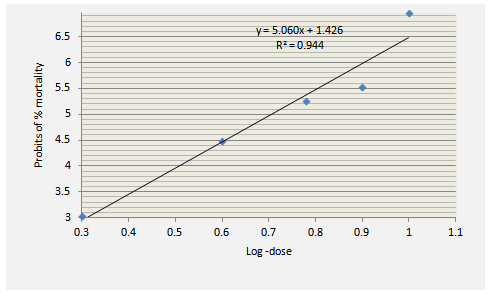 | Figure 1. Plot for the calculation of LD50 of N. laevis leaf extract |
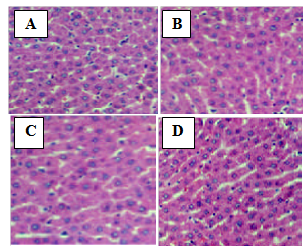 | Figure 2. Photomicrograph of the liver of rats treated with the extract of N. laevis (magnification x200). (A) Normal control; (B) 150 mg/kg; (C) 300 mg/kg; (D) 500 mg/kg |
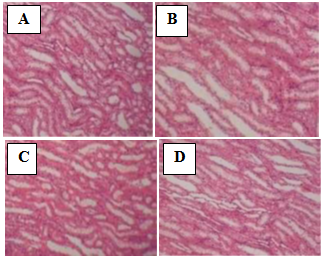 | Figure 3. Photomicrograph of the kidney of rats treated with the extract of N. laevis (magnification x 200). (A) Normal control; (B) 150 mg/kg; (C) 300 mg/kg; (D) 500 mg/kg |
|
|
|
|
|
|
4. Discussion
- Traditionally, herbal products are considered non-toxic and have been used by general public and traditional healers worldwide to treat various ailments. However, the fact that an herbal preparation is of natural origin does not necessarily make it safe. The active ingredients of plant extracts are chemicals like those of synthetic or purified drugs. In low amounts, they may be ineffective, while in the right amounts, they may prove beneficial. When large quantities are used and for a prolonged period, plant extracts may be injurious to health. They have the potential to cause serious toxic effects[2]. Therefore in this study, the acute and sub-chronic toxicity studies of the ethanolic extract of the leaves of Newbouldia laevis were carried out. These toxicity studies in animals are usually necessary for any drug intended for human consumption. The information obtained from such studies could be used to estimate the therapeutic index of drugs. These studies are also useful in selecting doses for chronic toxicity studies and providing preliminaryidentific- ation of target organs of toxicity [18]. In the acute toxicity study, adverse reactions such as sedation, reduced motor activity, unresponsiveness to light touch and auditory stimuli were observed only when the dose of the extract was increased above 1000 mg/kg body weight (results not shown). The LD50 (dose of the extract that caused 50 % mortality in the animals) was calculated from the dose-response curve as 5400 mg/kg. This suggests that the extract was non-toxic according to a toxicity classification [19]. The toxic effects observed at very high doses was likely caused by the chemical constituents of the leaves of N. laevis such as tannins, saponins, terpenes and flavonoids [10]. In the sub-chronic toxicity study, there was no mortality or any toxic manifestations observed at any of the doses selected throughout the study period. Changes in body and organ weight are valuable indicators for predicting the toxicity of a compound or plant extract [20, 21]. The significant reduction in body weight of the animals observed after one week of treatment with the extract could be normal physiological and adaptational responses media- ted by the extract through suppression of appetite [22]. These probably explain the observed reduction in food and water intake during the first week of toxicity study. However the weight subsequently increased suggesting that the extract did not exert adverse effects on the treated animals. The absence of significant changes in the organs except the spleenseems to suggest that ingestion of the extract did not induce organ damage in the treated animals. The observed significant increase in spleen weight could be due to a rise in the activity of the hematopoietic system caused by the ethanolic extract. Histopathological examination of the liver and kidney in treated and untreated rats indicates that the extract is non-toxic at the doses and for the period of 28 days it was administered. Neither morphological changes norinflamma- tory cells were observed in the tissues during histopatholo gical assessment of liver and kidney samples at all the test doses.Estimation of blood parameters is crucial in evaluating the toxicity of drugs as changes in hematological system in animal studies have a high predictive value for human [23]. All blood parameters estimated in the treated rats, except the platelets did not show significant difference compared with the control group. However it should be noted that the levels of erythrocytes (RBC), leucocytes (WBC), hemoglobin (HB) and packed cell (PCV) slightly increased. The slight increase in these parameters and the significant increase observed in platelets may be due to stimulatory effect of the extract on the production of hematopoietic regulatory elements such as thrombopoietin, erythropoietin and colony – stimulating factors by the stromal cells and macrophages in the bone marrow [24]. The slight increase in WBC may also be due to the normal immunological reaction of the animals to foreign substances. There was no significant difference in the levels ofaspar- tate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) between extract-treated and control groups. ALT is a cytoplasmic enzyme found in very high concentration in the liver [5]. An increase in the serum level of this specific enzyme indicates hepatocellular damage. Although AST is less specific than ALT as a marker of liver damage, elevation in the serum levels of the two enzymes is an indicator of tissue damage and altered membraneperme ability [25] while alkaline phosphatase is a marker ofobstructive jaundice and intrahepatic cholestasis [26]. Administration of the extract did not cause any significant changes in the levels of these enzymes. This again indicates that the extract is not toxic to rats at the doses administered. In conclusion, the present study suggests that the ethanolic extract of Newbouldia laevis has low toxicity profile regarding hematological, blood biochemical and histological parameters in albino rats.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the technical support provided by Dr. J.O. Areola of the Department ofBiochemistry, Obafemi Awolowo University, Ile-Ife, Nigeria and Mr. I.O. Olaniran, College of Health Sciences, Ladoke Akintola University of Technology, Osogbo, Nigeria.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML