-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2013; 3(3): 44-50
doi:10.5923/j.ajmms.20130303.02
Characterization of Lipid Panel in Hiv-1 Infected Patients: A Study of Baseline and 52 Weeks Haart Patients
Osaretin Albert Taiwo Ebuehi, Kennedy Madaki Idoko, Anthony Ani
Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria
Correspondence to: Osaretin Albert Taiwo Ebuehi, Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The study is tocharacterize lipid panels with an emphasis on HDL-C (high density lipoprotein cholesterol) sub-fractions among HIV infected subjects in Lagos, Nigeria, in order to ascertain the pattern of alterations of these parameters in patients on HAART (highly active antiretroviral therapy) for 52 weeks. Serum concentrations of HDL-C and the HDL sub-fractions, HDL-C 2 and HDL-C 3, were measured in 20 HIV positive, HAART naïve and 20 HIV positive, first-line HAART experienced subjects and compared with those of HIV negative control group matched for sex and age.Serum total cholesterol (T-Chol) and low-density lipoprotein (LDL-C) levels were increased in HAART naïve patients and decreased in HAART experienced patients as compared to controls. Triglycerides and fasting blood sugar levels were significantly elevated in HAART naïve group in comparison with the other groups. Total HDL-C and HDL-C 3 concentrations were significantly lower in HAART naïve patients than in other groups while HDL-C 2 and HDL-C 2 / HDL-C 3 ratio in contrast, were not significantly different among all the groups. It is suggested that quantitation of HDL-C sub-fractions is not a valuable risk indicator for heart disease in HIV patients HAART naïve or experienced.
Keywords: HIV-1 Infection, HAART, Lipid Panels
Cite this paper: Osaretin Albert Taiwo Ebuehi, Kennedy Madaki Idoko, Anthony Ani, Characterization of Lipid Panel in Hiv-1 Infected Patients: A Study of Baseline and 52 Weeks Haart Patients, American Journal of Medicine and Medical Sciences, Vol. 3 No. 3, 2013, pp. 44-50. doi: 10.5923/j.ajmms.20130303.02.
Article Outline
1. Introduction
- High-density lipoprotein (HDL) is one of the five major groups of lipoproteins which are chylomicrons, VLDL, IDL, LDL, and HDL. These particles transport cholesterol and triglycerides within the water-based bloodstream. Those with higher levels of HDL-C seem to have fewer problems with cardiovascular diseases, while those with low HDL-C cholesterol levels (less than 40mg/dL or about 1mmol/L) have increased rates for heart disease1. While higher HDL levels are correlated with cardiovascular health, no incremental increase in HDL has been proven to improve health. The concentration value of HDL-cholesterol alone gives no information about the coronary risk of a patient. There are numerous reports that patients suffering from atherosclerosis and myocardial infarction have lower high-density lipoprotein (HDL) plasma concentrations as compared to control individuals2,3,4. High HDL levels thus point toward an active removal of the “atherosclerotic” VLDL and VLDL remnants. There are, however, many other theories discussed in that context. Since the hydrolysis of VLDL gives rise mainly to the HDL sub-fraction HDL-25, this latter lipoprotein class should mirror more precisely the activity of the lipolytic system than total HDL. The HDL-2 plasma concentrations thus may serve as a better risk indicator than total HDL. The serum concentration of several lipids, including high-density lipoprotein-cholesterol (HDL-C) and the HDL sub-fractions, HDL-2-C and HDL-3-C, were measured in 44 male and 26 female survivors of myocardial infarction and compared with those of a control group matched for age, sex, and body weight. Serum concentrations of total cholesterol (TC) and low-density lipoprotein (LDL-C) were significantly increased in patients as compared to control individuals. The total HDL-C concentration was lower in patients than in controls. By differential quantitation of HDL sub-fractions with a precipitation method using polyethylene glycol, it was found that HDL-3-C was not significantly different between female patients and controls. The reduction of HDL-3-C in male patients was only of borderline significance. HDL-2-C in contrast was significantly reduced in both male and female patients. The greatest difference between patients and controls was found in the HDL-2/HDL-3-C ratio. It is therefore concluded that HDL-2-C quantitation is a valuable risk indicator for myocardial infarction yielding a better discrimination of patients from controls than total HDL-C quantitation6. Protease inhibitors (part of HAART) have been shown to cause hyper- and dyslipoproteinemia. Since antiretroviral therapy is able to delay disease progression and possibly extend life expectancy in HIV-infected individuals, the precise nature of serum lipid disturbances may become of clinical interest with respect to its atherogenicity and to finding treatment options. Berthold et al., investigated prospectively, in 19 subsequent HIV-positive male patients (mean age 42±13 years), multiple lipid parameters in plasma, before and during treatment with a protease inhibitor (nelfinavir, ritonavir, or indinavir) and two nucleoside analogue reverse transcriptase inhibitors (NRTI). The median (range) treatment duration was 22 (7-40) weeks. 12 patients were treatment-naive; 7 had already NRTI medication at baseline. Their data indicate that the predominant feature of dyslipidemia under protease inhibitors is an increase in triglyceride-containing lipoproteins. This observation is in accordance with the hypothesis of increased apoptosis of peripheral adipocytes, release of free fatty acids and subsequent increased synthesis of VLDL. The lipid profile, based on the ratio of total cholesterol to HDL cholesterol and the ratio HDL2 to HDL3, is significantly more atherogenic7.
2. Materials and Methods
2.1. Research Subjects
- Human immunodeficiency virus (HIV) infected subjects on first line highly active antiretroviral therapy (HAART) for an average of 52 weeks; HIV infected subjects naïve to HAART all recruited at the APIN-PEPFAR (AIDS Prevention Initiative in Nigeria-President’s Emergency Plan for AIDS Relief) clinic of the Lagos University Teaching Hospital (LUTH), Idi-araba, Surulere, Lagos and non-infected human subjects. A minimum of 25 subjects for each group - HAART experienced, HAART naïve and non-infected human subjects (determined by the Cochran formula and a 4.1% prevalence rate) were involved in this study. All subjects were at least 18 years old as at the time of this study. Gender balance was taken into consideration. Also, no form of financial or material inducement was given for subjects’ participation in this study.
2.2. Experimental Procedure and Sample Collection
- 8ml whole blood samples were collected in lithium heparin and fluoride oxalate vacutainers from volunteer HAART experienced (as indicated by their patient registration/PEPFAR identities and data, visit to clinicians, and collecting ARVs at the APIN-PEPFAR clinic) and naïve HIV positive (as confirmed by the HIV screening done in APIN-PEPFAR clinic using the serial testing algorithm) subjects in the research location site, while sampling for volunteer HIV negative subjects within LUTH and CMUL was done after a serial screening algorithm using determine HIV-1 rapid test kit showed a negative result. The process of specimen collection brought some slight discomfort to the patients but nothing was done outside the normal management of the patient. However, the benefits of this project outweigh the risk. Necessary bio-safety level facilities required for major stages in the research were put to use. In addition, laboratory standard operating procedures were strictly adhered to.
2.3. Blood Chemistry
2.3.1. HDL And Sub-Fractions
- The exact procedure of this method has been published Kostner et al.,8: Quantolip consists of two reagent solutions, A and B with 9.5% and 15% polyethylene glycol (PEG) dissolved in 0.1mmol phosphate buffer, and 0.9g/l sodium azide, respectively; 100µl of serum/plasma samples are mixed with 200µl solutions A or B respectively, and centrifuged in an Eppendorf centrifuge. Solution A precipitates all Very Low Density Lipoprotein Cholesterol (VLDL-C) and Low Density Lipoprotein Cholesterol (LDL-C), and total HDL-C remains in the supernatant. Solution B precipitates VLDL, LDL, and HDL-C 2, leaving HDL-C 3 in the supematant. A 3µl measure of the supernatant from reagent A and 3µl supernatant from the reagent B were used to assay for total cholesterol. The content of HDL-C 2 was obtained by calculating the difference between total HDL-C and HDL-C 3 subclass as described in the Quantolip kit.
2.3.2. Total Cholesterol
- The Roeschlau and Allain enzymatic method of 1974 was used9,10. This is based on the determination of ∆4-cholestenone after enzymatic cleavage of the cholesterol ester by cholesterol esterase, conversion of cholesterol by cholesterol oxidase, and subsequent measurement by the Trinder reaction of the hydrogen peroxide formed11.
2.3.3. Triglycerides
- This is based on the work by Wahlefeld using a lipoprotein lipase (LPL) from microorganisms for the rapid and complete hydrolysis of TGs to glycerol followed by oxidation to dihydroxyacetone (DHAP) and hydrogen peroxide under the catalysis of glycerol kinase (GK) and glycerol phosphate oxidase (GPO)12 and also subsequent measurement by the Trinder reaction of the hydrogen peroxide formed11.
2.3.4. LDL-Cholesterol
- This was done using the Friedewald equation13 by subtracting the amount of cholesterol associated with HDL and VLDL assuming a prolonged fasting state (12 – 14hours): Total Cholesterol – Total HDL-C – (0.45 X TG) = LDL-C, if all the quantities are measured in mmol/L.
2.3.5. Glucose
- This hexokinase method based on the work of Schmidt, Peterson and Young was used14,15. Hexokinase catalyzes the phosphorylation of glucose to glucose-6-phosphate by ATP. Glucose-6-phosphate dehydrogenase oxidizesglucose-6-phosphate in the presence of NADP+ to gluconate-6-phosphate. The rate of NADPH formation during the reaction is directly proportional to the glucose concentration and was measured photometrically at 340nm.
3. Stastistical Analysis
- Statistical analysis was performed using commercially available software (Prism for Windows, version 5.00; GraphPad Software). All data were expressed as mean ± standard error of the mean (SEM). Inter-group differences were analyzed using one-way ANOVA for the comparison of three or more groups and values were considered significant at p<0.05.
4. Results and Discussion
4.1. High Density Lipoprotein Cholesterol (HDL-C) and sub-fractions
- The results of High Density Lipoprotein Cholesterol (HDL-C) and sub-fractions are presented in Figure 1. There was a significant elevation of HDL-C and HDL-C 3 for the Human Immunodeficiency Virus positive/infected, Highly Active Anti-Retroviral Therapy positive/experienced (HIV +VE HAART +VE) group as compared with the HIV +VE HAART –VE group while no significant variation was observed as compared with the HIV –VE group. No significant variation was also observed for the HDL-C 2 sub-fraction and HDL-C 2:HDL-C 3 ratio among the three groups. The only significant variation for the HIV –VE and the HIV +VE HAART –VE groups is in the HDL-C 3 concentration.The activity of cholesterol ester transfer protein (CETP), which transfers cholesterol esters from HDL-C to apolipoprotein-B containing proteins16, is elevated in HIV infection, and its activity correlates inversely with serum HDL-C concentrations17. This may help explain why HDL-C levels are lower in the HIV +VE HAART –VE subjects. Although the reason for elevated CETP activity is still to be determined, CETP functions more efficiently in the setting of high TG levels18, and this could help explain the increased activity in the HIV +VE HAART –VE subjects who also have significantly (p<0.001) elevated TG levels when compared with the other study groups as shown in Figure 2. The pattern of HDL-C and its sub-fractions concentration for the three study groups is similar i.e. there is no discrimination whatsoever because the HIV +VE HAART +VE group which had the highest HDL-C concentration also had the highest values of the sub-fractions (although not significantly different from the HIV –VE group) while the HIV +VE HAART –VE group with the least HDL-C concentration had it low also for the sub-fractions in line with the report by Grunfeld et al. that HDL-C levels are reduced in untreated HIV infection suggesting that HIV itself apart from HAART may also increase cardiovascular disease risk19. In HIV +VE individuals who start HAART that effectively suppresses HIV replication, HDL-C levels increase, regardless of whether a protease inhibitor is used20,21,22, implying that the HIV effect on HDL-C levels is greater than the HAART effect. The HIV –VE group was somewhere mid-way between the two extremes. This does not go with the conclusion of Brugger et al. that HDL-C 2 quantitation is a valuable risk indicator for myocardial infarction yielding a better discrimination of patients from controls than total HDL-C quantitation6. It rather seems to agree with the findings of Sweetnam et al. that both HDL-C 2 and HDL-C 3 (which are a reflection of HDL-C concentration) are inversely associated with incidence of heart disease because the prediction of the risk of heart disease from total HDL-C alone was not improved upon by measurement of the two HDL-C sub-fractions. The relative value of the two HDL-C sub-fractions as predictors of risk is still unresolved. The uncertainty may be due, at least in part, to problems associated with their measurement23.
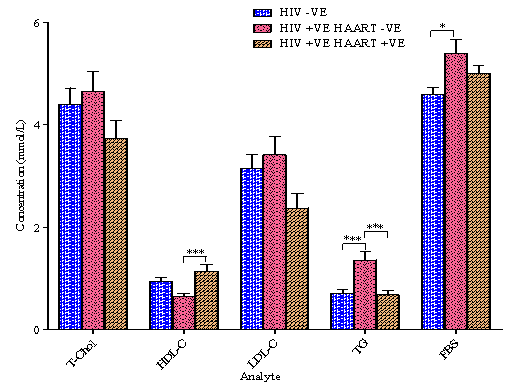
4.2. Lipid Panel and Fasting Blood Sugar (FBS)
- The results of lipid panel and fasting blood sugar (FBS) are presented in Figure 2. Only the triacylglyceride (TG) concentration of the HIV +VE HAART –VE is significantly elevated when compared with the other study groups while its FBS is significantly higher than only that of the HIV –VE group. No noteworthy variation was seen in the Total Cholesterol (T-Chol) and the Low Density Lipoprotein Cholesterol (LDL-C) levels among the three groups while only the HIV +VE HAART +VE group had a significantly higher HDL-C than that of the HIV +VE HAART –VE group.In HIV patients not treated with HAART, HDL-C levels are low and triglyceride levels are high19,24. The high triglyceride levels may be a result of decreased triglyceride clearance, which has been correlated with higher interferon-α levels19. Smoking also reduces HDL-C levels25, but none of the HIV +VE HAART –VE subjects in this study responded positively to “Do you smoke?” i.e. all are non-smokers. This observation is in accordance with the hypothesis of increased apoptosis of peripheral adipocytes, release of free fatty acids and subsequent increased synthesis of VLDL7. In a study by Schmitz et al., two major alterations in the metabolism of apoB – containing lipoproteins were identified as contributing to HAART associated dyslipidemia: first, a doubling of the rate of total apolipoprotein B synthesis, in particular of VLDL1 and VLDL2 apo B, and second, a decrease of the transfer rate of triglyceride-rich VLDL1 to denser more cholesterol ester-rich VLDL2 by more than 80%26. However, the observed differences between metabolic parameters are not necessarily attributable in total to the effects of HAART alone but may also be assigned to some extent to HIV infection itself.
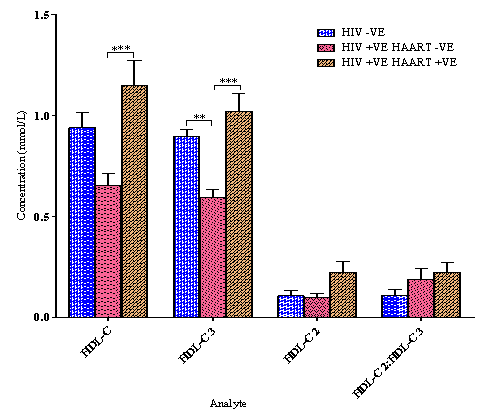 | Figure 1. HDL-C and sub-fractions mean values (±SEM). Brackets indicate differences between groups as determined by a 1-way ANOVA with Tukey's Multiple Comparison Test (* *p<0.01, * * *p<0.001) |
5. Conclusions
- With the lack of tangible indicators from HDL-C sub-fractions, total HDL-C is sufficient in assessing the risk of cardiovascular diseases especially in low income settings like ours. The assemblage of increased T-Chol chiefly the LDL-C sub-fraction and total triglycerides, together with low HDL-C levels and reduced insulin sensitivity as can be inferred from fasting hyperglycemia constitutes a highly atherogenic risk profile for HIV patients indistinguishable from that observed in patients with the metabolic syndrome. A beyond question appraisal of the impact of HAART on the risk for cardiovascular events demands prospective studies with a longer follow-up and more patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML