-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2013; 3(2): 27-37
doi:10.5923/j.ajmms.20130302.03
Artemisinin Based Combination Versus Micronutrient Combination in Malaria Therapeutics: A Randomized Controlled Clinical Trial
Iribhogbe O. I.1, Agbaje E. O.2, Oreagba I. A.2, Aina O. O.3, Emordi J. E.1, Akahomen J. E4, Nmorsi O. P. G.5
1Department of Pharmacology & Therapeutics College of Medicine, Ambrose Alli University Ekpoma
2Department of Pharmacology, College of Medicine of the University of Lagos
3Malaria Research Unit, Nigerian Institute of Medical Research, Yaba Lagos State
4Faith-dome Medical Centre, Ekpoma Edo State
5Parasitology Unit, Department of Zoology, Ambrose Alli University Ekpoma, Edo State
Correspondence to: Iribhogbe O. I., Department of Pharmacology & Therapeutics College of Medicine, Ambrose Alli University Ekpoma.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This randomized controlled clinical trial was conducted to elucidate the role of some selected antioxidant micronutrients in the course and therapeutics of malaria. In this study, 150 participants (6 months- 5 years) were recruited for the study and were randomized into 15 cohorts of 10 patients each. The active comparator group or control groups were administered with oral doses of standard artemisinin based combination therapy, while the interventional cohorts were administered varying combinations of antimalarials (Artesunate or Amodiaquine) and micronutrients (vitamin A, vitamin E, zinc and selenium). Results showed that a more rapid parasite clearance time (PCT) was observed in the Artesunate + vitamin A + zinc treated group (26.00±4.82 hours) when compared with the active comparator groups (Amodiaquine + Artesunate; 27.00±3.00 hours and Artemether + Lumefantrine; 29.33±3.53 hours) which was not significant between groups (F = 0.93; P > 0.05). Conclusively, antioxidant micronutrients have potential benefit as adjuvants in the management of uncomplicated falciparum malaria in children.
Keywords: Combination Therapy, Adjuvant Therapy, Antioxidant Micronutrients
Cite this paper: Iribhogbe O. I., Agbaje E. O., Oreagba I. A., Aina O. O., Emordi J. E., Akahomen J. E, Nmorsi O. P. G., Artemisinin Based Combination Versus Micronutrient Combination in Malaria Therapeutics: A Randomized Controlled Clinical Trial, American Journal of Medicine and Medical Sciences, Vol. 3 No. 2, 2013, pp. 27-37. doi: 10.5923/j.ajmms.20130302.03.
Article Outline
1. Introduction
- A recent estimate of malaria incidence derived from routine surveillance data suggest 174 million episode occurred in African region in 2011 with estimated deaths of 596, 000[1].Malaria has been estimated to cause 2.3% of global disease and 9% of disease in Africa[2]; it ranks third among major infectious disease threats in Africa after pneumococcal acute respiratory tract infections (3.5%) and tuberculosis (TB) (2.8%). The estimated annual direct and indirect cost of malaria was 800 million US dollars in 1987, this was expected to exceed 1.8 billion US dollars by 1995[3]. The vast majority of malaria deaths occur in Africa, south of the Sahara, where malaria also presents major obstacles to social and economic development. Malaria has been estimated to cost Africa more than 12 billion US dollars every year, even though it could be controlled for a fraction of that sum. The interaction between malaria and nutrition is complex[4] and has been the subject of controversy since the early 1950s. Several studies have shown associations between malaria and protein energy malnutrition, poor growth and certain micronutrient deficiencies among children[5]. Despite clear evidence of the impact of malaria on the nutritional status of affected individuals, the effect of nutritional status on host resistance to the acquisition and progression of malaria is still not clearly defined. A more recent review of the malaria–nutrition literature[6],[7], concluded that the earlier findings of a protective effect of malnutrition against malaria were mainly based on studies with several methodological shortcomings. Reappraisal of the data together with recent literature indicates that the effect of nutrition on host susceptibility to malaria is more complex and, in many cases, poor nutritional status predisposes the host to an increased risk of infection, symptomatic clinical malaria attacks, and a higher likelihood of mortality from malaria[7]. Five studies conducted in Madagascar[8], Nigeria[9], Chad[10], The Gambia[11], and Senegal[12] indicate that malnourished patients are 1.3–3.5 times more likely to die or have permanent neurological sequelae than normally nourished malaria patients. Depletion of plasma borne pro-vitamin A carotenoids during acute malaria attacks has been described[13]. A placebo-controlled trial of zinc supplementation of pre-school children in Papua New Guinea and Burkina Faso provides additional evidence for the role of zinc in malaria[14],[7]. No human studies have addressed the role of selenium in malaria. Vitamin C has also been studied in animals but there have been few human studies. Other reports suggest that persons with lower plasma vitamin E levels recover more quickly from clinical malaria[15]. A more recent report revealed that intraperitoneal administration of vitamin E negatively impacted on the course of P. berghei development in mice[16] and that vitamin C deficiency in an L-gulono-γ-lactone oxidase gene knockout mouse might not affect the development of malaria parasite in mice[17]. Essentially, the present study is designed to evaluate the effect of selected antioxidant micronutrients when used in varying combinations as adjuvants in the treatment of uncomplicated falciparum malaria as well as determine the antimalarial effect of selected standard agents when used in combination with selected micronutrients in the therapy of uncomplicated falciparum malaria in early childhood.
2. Methods
2.1. Study Area
- The study was conducted in Ekpoma, Esan West Local Government Area of Edo State Nigeria. This community is a semi-urban community with an estimated population of over 125,842 inhabitants[39]. Patients were recruited from two Medical Centers; Central Primary Health Center and Faith-Dome Medical Center both in Esan West Local Government Area of Edo State.
2.2. Sample Size Estimation
- Sample size was estimated at 5% significance and 80% power using the method of Campbell et al.,[40]. The formulae M = 2 x[Z (1-α/2) + Z (1-β)] 2 ÷ Δ2 and Δ= P1-P2/√p x (1-p) was used for sample size estimation. Where p= P1+P2/ 2, Z (1-α/2) = 5% = 0.05 = 1.96, Z (1-β) = power at 80%=0.8=0.8416 and Δ= standardized difference = 2.1053. Therefore, the minimum sample size is approximately 5 patients per group (total of 75 participants). Sample size was increased to 150 to make room for lost to follow up.
2.3. Study Participants
- A total of 150 participants were recruited for the study. The participants were drawn from early childhood (6months-5yrs of age).
2.4. Study Design
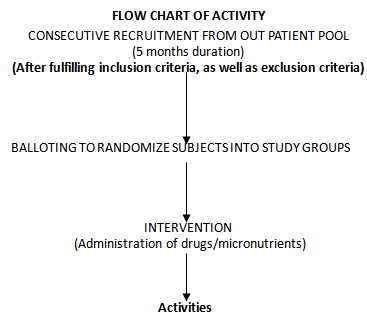
- The study is a randomized controlled clinical trial with consecutive recruitment of eligible patients until the total sampling size was achieved. Specifically designed medical record forms were used to elicit biodata and clinical data from participants. Participants were admitted into the study after meeting the following inclusion criteria:● age of ≥ 6months ≤ 5 years,● asexual parasitemia of between 1,000 and 200,000/μl,● acute manifestation of malaria (e.g., history of fever in the preceding 24 hours, a temperature of >37.5°C at baseline),● body weight between 5 and 30 kg,● ability to tolerate oral therapy,● informed consent by the legal representative of the participant (the parents, if possible), oral agreement of the child if appropriate.● resident in the study area for duration of at least 4 weeks.The exclusion criteria for the study were as follows:● adequate antimalarial treatment within the previous 7 days,● use of micronutrients in the last 2 weeks,● use of herbal medications in the last 2 weeks ● antibiotic treatment for a concurrent infection● haemoglobin level of <7 g/dl,● haematocrit of <25%,● leukocyte count of >15,000/μl,● mixed plasmodial infection,● severe malaria, any other severe underlying disease,● concomitant disease masking assessment of the treatment response,● inflammatory bowel disease and any other disease causing fever.
2.5. Ethical Issues/Considerations
- The trial was registered with clinicaltrials.gov (registration number NCT01152931) and was conducted in accordance with the principle of Helsinki Declaration and its Hong Kong amendment and according to the principle of good clinical practice. Ethical permission was obtained from the Ethical Review Board of the Edo State Ministry of Health after submitting the research proposal for the study. Informed consent was procured from Parents and Guardian of study participants and or directly from the participants depending on their age group. The participants on micronutrient combinations without an appropriate response were scheduled to be given adequate treatment with artemisinin based combination therapy after 72 hours of commencing micronutrient therapy.
2.6. Study Drugs and Administration
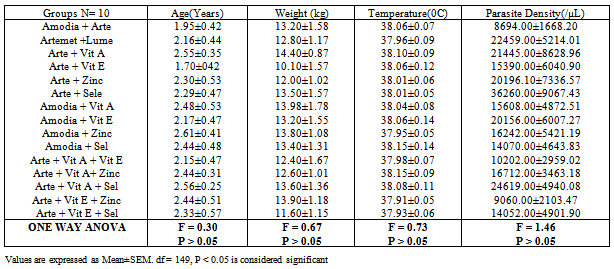
- Drugs were administered daily at different doses depending on the age and weight of the participants. The intervention was based on the use of standard antimalarial combination therapy for uncomplicated malaria according to WHO recommendation[41]. Micronutrient dosage was based on dietary reference intake values (DRI) adapted from Food and Nutrition Board (FNB: IOM)[42]. The study participants were randomly grouped into 15 cohorts (A-O) of 10 patients each after appropriate age and sex matching. Envelopes containing the letters (A1-10, B1-10, C1-10, D1-10, E1-10, F1-10, G1-10, H1-10, I1-10, J1-10, K1-10, L1-10, M1-10, N1-10 and O1-10) were placed in a basket. The content of the envelope picked after balloting, determined the arm of the study the participants were allotted to. The treatment groups are shown in Table 1.
2.7. Study Flow and Procedures
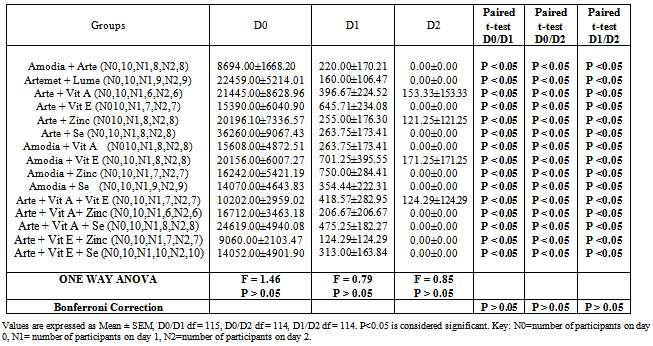
- Patients were recruited consecutively for 5 months (August to December, 2010) until the sampling size was achieved. The study is a single blind study with concealment of the interventional groups using the sealed envelope system[43]. The patients were seen by a study physician at 24-hours intervals after each drug administration and/or until two consecutive negative blood smears occurred and subsequently on days 7, 14, 21, and 28 post-treatment or as otherwise indicated. Adverse events were monitored and documented accordingly during the follow-up period. Venipunctures were performed on study days 0 and 28 to monitor the hemoglobin level, hematocrit, and differential white blood cell count.
|
 D0 = administration of study drugs/micronutrients under direct observation of physicianDI = follow up activities (history, physical examination, preparation of blood smears, adverse event monitoring etc)/administration of drugs/micronutrientsD2= same activity as aboveD3 = same activity as aboveD4=5th day of event = history taking, physical examination, preparation of blood smears, adverse event monitoring etc.7th day = same activity as above14th day = same activity as above21st day = same activity as above28th day = same activity as above----- End of follow up activityNote: Recruitment, intervention and follow up was done consecutively for the subjects over 5 months although each patient was followed up for 4 weeks. This implies that subjects had different D0-D4 and different 7th, 14th, 21st and 28th day of follow up.Lost to follow up occurred predominantly on day 2. Reason presented by the parents/guardian of subject is the pain of subjecting their wards to finger prick for blood smear preparation.Key: D0-D4 = Days of drug/micronutrient administration
D0 = administration of study drugs/micronutrients under direct observation of physicianDI = follow up activities (history, physical examination, preparation of blood smears, adverse event monitoring etc)/administration of drugs/micronutrientsD2= same activity as aboveD3 = same activity as aboveD4=5th day of event = history taking, physical examination, preparation of blood smears, adverse event monitoring etc.7th day = same activity as above14th day = same activity as above21st day = same activity as above28th day = same activity as above----- End of follow up activityNote: Recruitment, intervention and follow up was done consecutively for the subjects over 5 months although each patient was followed up for 4 weeks. This implies that subjects had different D0-D4 and different 7th, 14th, 21st and 28th day of follow up.Lost to follow up occurred predominantly on day 2. Reason presented by the parents/guardian of subject is the pain of subjecting their wards to finger prick for blood smear preparation.Key: D0-D4 = Days of drug/micronutrient administration 2.8. Therapeutic End Points
- The primary efficacy end point for the study was the 7-day cure rate[41]. The primary safety end point in the study was the emergence of adverse events after the start of treatment. Secondary end points were the parasite and fever clearance time, recrudescence time and the 28 day cure rate.
2.9. Laboratory Procedures
- Dried thick blood smears were stained with 10% Giemsa solutions at pH 7.2 for 10 minutes. Parasite species were identified using standard morphological characteristics, and the parasite density was calculated using standard procedure in which parasite were counted per 200 WBC multiplied by a standard count of 8,000 leukocytes/μl[44].
2.10. Data Management and Statistical Analysis
- Statistical analyses of the data were performed using statistical software package SPSS version 17.0. Cure rates were calculated from the number of patients with clinical and parasitological cure by day 7, 14, or 28 divided by the total number of patients who could be evaluated (per protocol population)[41]. Fever clearance time (FCT) was calculated from the start of treatment until the first of two consecutive temperature measurements remained below 37.5°C[44]. The time required for parasite clearance (PCT) was calculated as the time between the beginning of treatment and the time when no asexual forms were found in the blood film. The parasite reduction ratio or rate was calculated as the rate between the parasite density before treatment and that at 48 hours, as described by[44]. The safety analysis includes abnormal laboratory data and adverse events for all subjects who received at least one dose of the study drug (intention-to-treat population). Student's t test and one way ANOVA were used to compare the mean of laboratory data between groups. Bonferroni correction was done as post- hoc test for multiple comparisons. The statistical significance level was set at 95% confidence interval and P value < 0.05 was considered significant.
3. Results
- Of the 150 participants recruited for this study, only 116 (77.33%) were successfully and completely followed up over a period of 4 weeks. As shown in Table 2, the mean peak age of the participants in the study was 2.31±0.11 years.
|
|
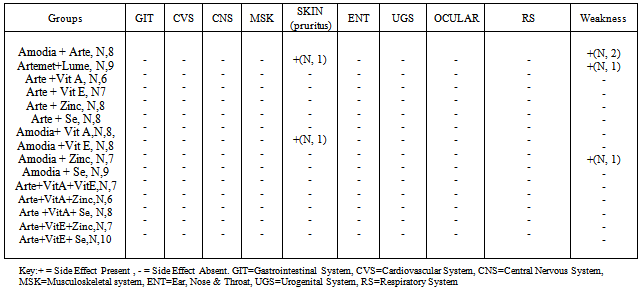
 | Figure 1. Frequency of Malaria Related Symptoms in Participants |
|
|
 | Figure 2. Parasite Clearance rate in Study Participant |
|
4. Discussion
- Malaria commonly afflicts populations that are both impoverished and malnourished, and a large proportion of the burden falls upon children. The antimalarial activity of vitamin A as demonstrated in this study is supported by the study of Hamzah et al.,[18] which revealed that retinol inhibits the growth of cultured Plasmodium falciparum. A study conducted in Calabar by Akpotuzor et al.,[14], revealed that there was a significant reduction in the level of total antioxidants and vitamin A which correlated strongly (negatively) with the severity of falciparum malaria; this signifies that antioxidant vitamins may have potential benefit in malaria therapeutics. Vitamin A supplementation in pre-school children is known to decrease the risk of mortality and morbidity from diarrhoea, measles, HIV infections and malaria. These effects are likely the action of vitamin A on immunity. The immunomodulatory role of vitamin A has been described in clinical trials and can be correlated with outcome of supplementation[19]. Vitamin A is an important nutrient required for maintaining immune function, playing an important role in humoral antibody responses[6],[20],[21]. In another study conducted in Kampala Uganda by Amy et al.,[22], parasite clearance in children with uncomplicated falciparum malaria was associated with elevated levels of antioxidants (vitamin A, B-carotene, Lycopene and vitamin E). This study suggests that children with acute malaria have depressed plasma concentration of antioxidants and that an increased concentration of antioxidants including vitamin E was associated with more rapid clearance of malaria parasite. The clinical trial presented in this work appears to be the first clinical trial to demonstrate the beneficial effect of selenium as an adjuvant in the management of acute uncomplicated falciparum malaria in preschool children. Findings from the present study revealed that selenium, when used as an adjuvant to artesunate and amodiaquine as well as in the presence of other micronutrients demonstrates a remarkably beneficial antimalarial activity. This is supported by a rapid parasite and fever clearance time in all the groups supplemented with selenium and a 100% 7 day and 28 day cure rate in all the groups supplemented with selenium. Other studies reveal that zinc is essential for a variety of lymphocyte function implicated in resistance to malaria including production of Immunoglobulin G, IF-γ, TNF-α and enhances the microbicidal activities of macrophages[23]. However, a recently published clinical trial, using combined vitamin A and zinc supplementation in young children with uncomplicated falciparum malaria in Burkina Faso, showed a major reduction in malaria morbidity in the supplemented group (34%) compared with placebo (3.5%) p<0.001. This finding corroborates the present finding which revealed that artesunate + vitamin A + zinc combination had a more rapid parasite clearance (26.00±4.82 hours) when compared to the active comparator groups (27.00±3.00 hours and 29.33±3.53 hours respectively). Fever clearance time (22.00±2.00 hours) was not significantly different (F = 1.02; P >0.05) from the active comparator groups. Similarly, a comparatively rapid fever clearance was also observed in the artesunate + vitamin E + zinc combination group 22.29±4.85 hours. In the Burkina Faso study, time to first malaria episode was higher in the supplemented group. The supplemented group also had 22% fewer fever episodes when compared to the placebo group[7]. Three zinc supplementation trials have been done so far. Studies in The Gambia and Papau New Guinea showed reduction of about one third (38%) reduction in the rate of visit to a health facility for a clinical syndrome consistent with malaria and confirmed by parasitological examination of the blood[14]. The third trial was done in Burkina Faso by Müller et al.,[24]. The study found no effect of zinc supplementation on the rate of malaria episode as ascertained from house hold visits. These results are however, conflicting and inconclusive. In a study by Duggan et al.,[25] plasma zinc concentration was found to be depressed during the acute phase response in children with falciparum malaria. According to a report from a randomized control trial by Zinc Against Plasmodium Study Group in 2002[26], children between the ages of 6months – 5 years with fever and asexual parasitemia were placed on zinc supplements (20mg/day for infants, 40mg/day for older children) for 4 days. Results obtained showed no significant effect of zinc on the median time to reduction of fever, no significant reduction in parasitemia and no significant change in Haemoglobin concentration in the 3 day period of treatment and 4 weeks period of follow up. Plasma zinc levels were found to be consistently low in all the children at base line. Hence the author concluded that zinc does not appear to have a beneficial effect in the treatment of acute uncomplicated malaria in preschool children. Children less than 5 years old are at increased risk of protein energy malnutrition (PEM), as well as deficiencies in micronutrients including zinc[27]. Zinc deficiency in humans leads to growth retardation , thymic atrophy, lymphopenia, impaired T and B cell function, impaired chemotactic activity of neutrophils and a reduction in thymic activity, interferon-γ concentration and the number of CD4+ lymphocytes[23]. These alterations in the cellular and humoral functions may increase host susceptibility to Plasmodium falciparum [23],[28],[29]. Zinc supplementation in developing countries has resulted in improvement in delayed cutaneous hypersensitivity[30] and an increase in CD4+ lymphocytes[31]. Zinc supplementation has been found to reduce the incidence of diarrhoea and pneumonia[32] and to be beneficial when used as adjunctive therapy for acute diarrhoea[33],[34]. This is in consonance with results from this study which showed a remarkable recession of diarrhoea in patients who received zinc supplementation in addition to other antimalarials. The community based zinc supplementation trial in the Gambia showed a reduced health centre attendance for malaria in children who received zinc[35]. In Papau New Guinea, zinc supplementation in pre-school children reduced malaria attributable hospital attendance[23]. This is in agreement with the findings of the present study which revealed a more rapid parasite clearance time (PCT) in the Artesunate + vitamin A + zinc treated group (26.00±4.82 hours) when compared to the active comparator groups (Amodiaquine + Artesunate; 27.00±3.00 hours and Artemether + Lumefantrine; 29.33±3.53 hours). Zinc and vitamin A interact in several ways; zinc is a component of retinol binding protein a protein necessary for the transporting of vitamin A in the blood. It is also important in activating the enzyme that converts retinol to retinal[36]. Till date, only one clinical trial has been done to evaluate the effect of zinc as an adjunct in the treatment of acute, uncomplicated falciparum malaria in under five’s. In this study the children received zinc supplements at 20mg/day for infants and 40mg/day for older children for 4 days in addition to chloroquine[26]. The results were quite disappointing as the result showed no beneficial effect when used as an adjunct to chloroquine in the management of uncomplicated falciparum malaria in pre-school children. However, this finding contravenes the present finding which revealed that zinc when used as adjuvant to artesunate and amodiaquine demonstrated beneficial antimalarial activity in acute uncomplicated malaria in under five or pre-school children. This is evidenced by; the occurrence of a more rapid parasite clearance time in the artesunate + vitamin A + zinc combination group when compared to the active comparator groups, the occurrence of a rapid fever clearance in all the zinc supplemented groups when compared with the active comparator group and a 7 day cure rate of 100% in all the zinc supplemented groups. In a placebo controlled trial by Villamor et al.,[37], using different vitamin combinations (vitamin B, C and E, multivitamins, vitamin A and β- carotene) were found to significantly reduce the incidence of clinical malaria by 71% while vitamin A, B and C multivitamins caused a non significant 63% reduction in malaria incidence in children born to HIV positive mothers. The effect of vitamin A on clinical malaria could be mediated in part by increased phagocytosis of infected red cells and decreased pro-inflammatory activity[38].
5. Study Highlights
- This study has revealed the following:• Combination of antioxidant micronutrients (vitamin A, E, selenium and zinc) with standard antimalarials may have immense therapeutic benefit as adjuvants in the management of uncomplicated falciparum malaria in early childhood.• Artesunate + vitamin A + zinc combination therapy has been shown to cause more rapid parasite clearance than standard artemisinin based combination therapy. Hence, we advocate for its use in malaria therapeutics as a means of providing a low cost therapeutic option in resource poor setting.AcknowledgementsWe acknowledge the contribution of all the authors whose unalloyed commitment ensured the successful completion of this work. Our warm regard and appreciation also goes to the Postgraduate School University of Lagos, Edo State Ministry of Health, Esan West Local Government Council and the Medical Director of Faith Dome Medical Centre, Ekopma Edo State.Conflict of Interest DeclarationAuthors hereby declare no conflict of interest in the research work conducted.Author’s ContributionDr Iribhogbe O.I was responsible for the design, development of the conceptual frame work and execution of the research. Drs Agbaje E.O, Oreagba I.A and Prof. Nmorsi OPG supervised the research work and ensured quality control. Drs Aina O.O, Akahomen and Emordi provided logistic support. Data analysis and manuscript preparation was done by Dr Iribhogbe O.I. All authors proof-read and contributed to the intellectual content of the manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML