-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2012; 2(6): 144-148
doi: 10.5923/j.ajmms.20120206.06
Microhaematuria and Proteinuria Performance as a Measured by Urine Reagent Strips in Estimating Intensity and Prevalence of Schistosomahaematobium Infection in Nigeria
Akyala Ishaku. A 1, Ashefo Daniel 1, Tanimu Habibu 1, Tsaku Mary 2, Agieni Ashem Godwin 3
1Department of science laboratory technology, College of Education, Akwanga
2Department of biological science, College of Education, Akwanga
3Dept of Microbiology,Kogi State University,Anyigba
Correspondence to: Akyala Ishaku. A , Department of science laboratory technology, College of Education, Akwanga.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Microhaematuria and proteinuria as measured by urine reagent strips are widely used to screen population at high risk of urinary schistosomiasis. This investigation was conducted to assess, if microhaematuria and proteinuria as measured by reagent strips could estimate intensity of Schistosomahaematobium infection in endemic areas and evaluate their screening performance among children in Benue State, Nigeria. A total of 1,124 urine samples were collected, screened for microhaematuria and proteinuria using reagent strips (Combi 9) and compared to filtration technique the gold standard method. A significant correlation was observed between microhaematuria (rho= 0.66, p<0.01), proteinuria (rho = 0.71, p<0.01) and intensity of Schistosomahaematobium eggs. Proteinuria had sensitivity of 95.7% and specificity of 67.7%, while microhaematuria had sensitivity of 64.8% and specificity of 89.6%. The proportion of false positive diagnoses was higher in proteinuria (19.6%) than microhaematuria (6.0%). The findings suggest that use of urine reagent strips could potentially estimate intensity of Schistosomahaematobium infection and their performance to screen urinary schistosomiasis agreed with previous observations.
Keywords: Microhaematuria, Proteinuria, False Positive, Benue State, Nigeria
Cite this paper: Akyala Ishaku. A , Ashefo Daniel , Tanimu Habibu , Tsaku Mary , Agieni Ashem Godwin , "Microhaematuria and Proteinuria Performance as a Measured by Urine Reagent Strips in Estimating Intensity and Prevalence of Schistosomahaematobium Infection in Nigeria", American Journal of Medicine and Medical Sciences, Vol. 2 No. 6, 2012, pp. 144-148. doi: 10.5923/j.ajmms.20120206.06.
Article Outline
1. Introduction
- Urinary schistosomiasis is a major debilitating disease caused by Schistosomahaematobium and characterized by the presence of blood in urine. Other symptoms are proteinuria, dysuria, bladder carcinoma, bladder stones, calcification of bladder wall and sometimes renal failure.The distribution of schistosomiasis has changed over time with some countries in South America, Asia, the Caribbean and the Middle East bringing down the prevalences of the disease through a concerted public health effort[1].In sub-Saharan Africa, prevalence levels have increased and vary from one country to another; this is mostly because of water resources development, roads and dams projects, irrigationof land for agricultural purposes, inactive control programme and mostly neglect from the part of governments to implement control programmes in endemic areas. Nigeria is one of the highly endemic countries where the disease has been unsystematically reported and large areas remain whose disease status is unknown[2].Screening using rapid, indirect tests has been proposed as a procedure to simplify mapping surveys[3]. Haematuria (blood in urine) has been proposed as a valid indication of current infection in Schistosomahaematobium endemic populations[2,4,5,6]. Testing urine with reagent strips for microhaematuria and proteinuria is such a simple and indirect diagnostic technique that could estimate the prevalence of urinary schistosomiasis in school children of endemic communities. Operational research studies in Africa showed that screening using reagent strips is an effective method to identify school children requiring treatment and subsequently monitor control[7,8,9]. Several research studies reported high sensitivity and specificity of reagent strips compared to urine filtrationconsidered the most conclusive diagnostic for urinaryschistosomiasis and this procedure is expensive, cumbersome and too technical for lay use[2,7]. However, this study was undertaken to assess if microhaematuria and proteinuria as detected with reagent strips could estimate intensity of Schistosomahaematobiuminfection in endemic areas and evaluate their diagnostic performance in screening urinary schistosomiasis among children in Benue State, Nigeria.
2. Materials and Methods
2.1. Study Area
- The study was conducted from November 2008 to September 2009 in Buruku and Katsina-Ala local government areas (LGAs) of Benue State, Nigeria which are known for their endemicity for urinary schistosomiasis [10,11]. Before the commencement of the study, permission was sought from Directors of health and local government education authorities of both areas. The climate of the areas is tropical with two seasons, the dry season which starts from October to March and the rainy from April to October. Agricultural activities like crop farming and rearing of animals are the mainstay of the inhabitants.
2.2. Sample Collection and Examination
- A total of 1124 urine samples were collected from pre and school children (primary and secondary) aged 3-27 years between 10:00 and 14:00 hrs using universal bottles. Urine were rapidly tested on the field using Medi Test combi 9 (Macherey-Nagel GmbH & Co.KG, Germany) reagent strips for the determination of microhaematuria and proteinuria. Microhaematuria and proteinuria were measured as erythrocytes/µl and mg/dl of albumin respectively. The degree of microhaematuriaand proteinuriaconcentrations were as follows: 0 (negative), Ca.5-10 (+), Ca.50 (++) and Ca.250 (+++) and 0 (negative), Ca.30 (+), Ca.100 (++) and Ca.500 (+++) respectively.Immediately after testing with the reagent strips, 1ml of ordinary household bleach was added to each collected urine sample to preserve any ova present and then taken to the laboratory within 2hrs for parasitological examination. 10 ml of urine was taken and filtered through a 12µm polycarbonate membrane in a filter holder. With the help of a forceps, the filter was removed from the filter holder and placed on a slide. A drop of Lugol’s iodine was added and the slide examined under microscope using x10 and x40 objective lenses. The number of eggs was counted per 10 ml of urine and intensities of infection were classified as 1-10 eggs, 11-49 eggs and > 50eggs for light, moderate and heavy infections respectively.
2.3. Statistical Analysis
- Collated data were double entered in Microsoft excel and analysed in PASW (Predictive Analysis software) version 18.0. Associations between variables were tested using Spearman correlation (rho) at p < 0.01 significance level.The diagnostic performance of microhaematuria and proteinuria was assessed by calculating sensitivity, specificity, positive predictive value and negative predictive value using the following formulae.● Sensitivity
 with a = True positiveb = False negative● Specificity
with a = True positiveb = False negative● Specificity 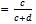 with c = True negatived = False positive● Positive predictive value (PPV)
with c = True negatived = False positive● Positive predictive value (PPV)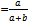 ● Negative predictive value (NPV)
● Negative predictive value (NPV)
3. Results
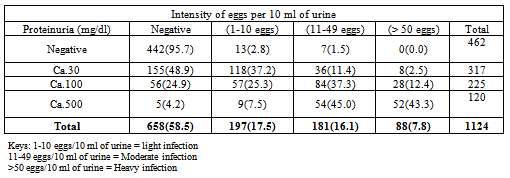
- Table1 shows the relationship between microhaematuria and intensity of Schistosomahaematobium eggs. Out of 151 having microhaematuria at Ca.5-10 (+), light infection had the highest rate 62 (41.1 %); Moderate and heavy infection had 39 (25.8 %) and 12 (7.9 %) respectively. Of the 72 screened for microhaematuria at Ca.50 (++), moderate infection had the highest rate with 42 (58.3%) while light and heavy infection 5(6.9 %) and 6 (8.3%) respectively. Of the 147 screened for microhaematuria at Ca.250, heavy infection had the highest rate with 69 (46.9 %). It was observed that various degree of microhaematuria concentrations, +, ++ and +++ corresponded to highest rateof light, moderate and heavy intensity of eggs respectively. A significant relationship was found between microhaematuria at different concentration and intensity of infection (rho = 0.66, p<0.01).The comparison betweenmicrohaematuria as indicator of urinary schistosomiasis and the true disease status as determined by filtration technique shows that microhaematuria was detected in 370 (32.9 %), among these302 (26.9 %) had both microhaematuria and presence of eggs (true positive) and 68(6.0 %) had microhaematuria with no presence of eggs (false negative). Of the 754 (67.1%) screened not havingmicrohaematuria in their urine, 164 (14.6 %) had Schistosomahaematobiumeggs (false positive) and 590 (52.5 %) were devoid of eggs (true negative).Table 2shows the relationship between proteinuria and intensity of Schistosomahaematobium eggs among children in Katsina-Ala and Buruku LGAs of Benue State. It was observed that of the 317 screened having proteinuria at Ca.30 (+), light infection recorded the highest rate with 118 (37.2 %), while moderate infection recorded the highest rate with 84 (37.3 %) out of the 225 screenedfor proteinuria at Ca.100(++). Heavy and moderate infection intensities recorded 52(43.3 %) and 54 (45.0%) respectively out of the 120 screened for proteinuria at Ca.500 (+++). It was observed that various degree of proteinuria concentrations, +, ++ and +++ corresponded to highest rateof light, moderate and heavy intensity of eggs respectively although moderate infection was found having an edge over heavy infection at Ca.500. A significant spearman correlation (rho= 0.71, p < 0.01) was found between different degrees of proteinuria concentrations and intensity of S. haematobium eggs. The comparison between proteinuria as indicator of urinary schistosomiasis and the true disease status as determined by filtration technique shows that proteinuria was observed in 662 (58.9%) children, 446(39.7%) had both proteinuria and Schistosomahaematobiumeggs (true positive), while 216(19.2 %) had proteinuria with absence ofS. haematobium eggs (false positive). Of the 442 screened having no proteinuria in their urine, 20(1.8 %) had S.haematobium eggs, while 442(39.3%) were devoid of S. haematobium eggs (true negative).The ability of microhaematuria and proteinuria to accurately identify all those with the disease (sensitivity) was 64.8% and 95.7% respectively, while their ability to correctly sort out all those without the disease (specificity) was 89.7 % and 67.2 % respectively.Microhaematuria had higher positive predictive value (PPV) (81.6 %) than proteinuria (67.7%), but had lower Negative Predictive Value (NPV) 78.2% against 95.6%.
4. Discussion
- The present study demonstrates that the use of microhaematuria and proteinuria to estimate the intensity of urinary schistosomiasis has potential utility in discriminating intensity of infection among infected individuals in endemic areas.The significant relationships observed betweenmicrohaematuria, proteinuria and filtration technique clearly demonstrate that the presence or absence of microhaematuria or proteinuria in urine is function ofSchistosomahaematobium eggs excretion in urine. However, the false positive results of microhaematuria and proteinuria observed entails the daily variation of S. haematobium eggs excretion in infected individuals. The absence of microhaematuria and proteinuria in infected individuals (false negative) could be the result of new infection in which tissues of the urinary bladder and kidney have not been damaged yet. The evaluation of only microhaematuria as indicator of urinary schistosomiasis shows sensitivity of 64.8 % and specificity of 89.6 %. This is closely related to findings of Ugbomoikoet al.[9] who reported sensivity of 68.3 % and specificity of 83.2 % among school children of two endemic areas in southwestern, Nigeria. However, the sensitivity of microhaematuria in this study is higher than that of Anosikeet al.[2] who obtained sensitivity of 41.0 % but with a similar specificity (82.0%) in a study conducted in Bende LGA of Abia State, Nigeria. Sensitivity and specificity of microhaematuria in this study are lower than that reported among zanzibari school children in Tanzania with sensitivity of 77.0%) and specificity of 97.0 %[8].However, variation in sensitivity and specificity of microhaematuria during Schistosomahaematobiuminfection has been reported in several studies conducted in different African settings. They have been reported to vary from 41.0 % to 93 % and from 67 % to 99 % for sensitivity and specificity respectively [2,4,6,8,9,12].
|
|
ACKNOWLEDGEMENTS
- Our sincere appreciation goes to all the children that participated in the study, their parents/guardians, teachers, head teachers and principals whose collaboration and understanding made the study feasible. We are indebted to Messrs J, Meme and C, Ansough, staff of the epidemiological units, Health Departments of Katsina-Ala and Buruku LGAs respectively for their help in translating English to local language during sample collection. We finally thank the Local Government chairmen of Katsina-Ala and Buruku Areas and their respective education authorities for granting permission before commencement of the research. This paper constitutes part of a PhD research of the first author to the Biological Sciences Programme, AbubakarTafawaBalewa University, Bauchi, Bauchi State, Nigeria. No grant was received for this research.
Conflict of Interest
- There is no any conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML