-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2012; 2(6): 136-143
doi: 10.5923/j.ajmms.20120206.05
Prognostic Contribution of the New Immunoglobulin (Ig) Biomarkers (Freelite™ and Hevylite™) in Waldenstrom’s Macroglobulinemia (WM)
M-C. Kyrtsonis 1, E. Koulieris 1, 2, D. Maltezas 1, T. Tzenou 1, S. Harding 3, E. Kastritis 2, N. Kafassi 4, V. Bartzis 1, A. Efthymiou 1, K. Bitsanis 1, M. Gavriatopoulou 2, E. Terpos 2, C Kalpadakis 1, M. K. Angelopoulou 1, T. P. Vassilakopoulos 1, A. R. Bradwell 5, Ph. Beris 1, G. A. Pangalis 1, P. Panayiotidis 1, M. A. Dimopoulos 2
1Haematology Unit of First Dpt of Propaedeutic Internal Medicine and Haematology Clinic, Athens Medical School Greece, Laikon Hospital
2Dpt of Therapeutics Alexandra Hospital, Athens Medical School, Greece
3The Binding Site Group Ltd PO Box 11712 Birmingham, B14 4ZB UK
4Dpt of Immunology, Laikon Hospital, Athens
5Dpt of Immunity and Infection Medical School University of Birmingham Edgbaston Birmingham, B15 2TT UK
Correspondence to: M-C. Kyrtsonis , Haematology Unit of First Dpt of Propaedeutic Internal Medicine and Haematology Clinic, Athens Medical School Greece, Laikon Hospital.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Clinical utility of serum free light chains (sFLC) and heavy chain (HLC) IgM were assessed, using Freelite and Hevylite assays in 70 WM patients. The result showed that median involved (i) sFLC -kappa and -lambda were 45.6 and 78.3mg/L respectively; median sFLC (kappa+lambda) was 67.4 mg/L. While, median FLCR (involved/uninvolved FLC ratios) were 2.7 and 6.9 in ikappa- and ilambda -restricted patients respectively. Moreover, increased isFLC, sFLC (kappa+lambda) and FLCR were correlated significantly with shorter time to first treatment (TFT) and adverse survival (OVS). Median iIgM-kappa and iIgM -lambda were 25.4 and 34.8g/L respectively; median HLCR (involved/uninvolved HLC ratios) were 185.5 and 101.9 in kappa- and lambda- restricted patients respectively. In addition, the increased iHLC and HLCR correlated significantly with shorter TFT. Hence, sFLC and HLC measurements appeared to be both prognostic in WM.
Keywords: Waldenstom’s Macroglobulinemia, IgM Quantification, Freelite™, Hevylite™, Monitoring, Prognosis
Cite this paper: M-C. Kyrtsonis , E. Koulieris , D. Maltezas , T. Tzenou , S. Harding , E. Kastritis , N. Kafassi , V. Bartzis , A. Efthymiou , K. Bitsanis , M. Gavriatopoulou , E. Terpos , C Kalpadakis , M. K. Angelopoulou , T. P. Vassilakopoulos , A. R. Bradwell , Ph. Beris , G. A. Pangalis , P. Panayiotidis , M. A. Dimopoulos , "Prognostic Contribution of the New Immunoglobulin (Ig) Biomarkers (Freelite™ and Hevylite™) in Waldenstrom’s Macroglobulinemia (WM)", American Journal of Medicine and Medical Sciences, Vol. 2 No. 6, 2012, pp. 136-143. doi: 10.5923/j.ajmms.20120206.05.
Article Outline
1. Introduction
- Waldenstrom’s macroglobulinemia (WM) is an indolent B cell lymphoproliferative disease, characterized by lymphoplasmacytic infiltration of the bone marrow (BM), lymph nodes or other organs accompanied by serum monoclonal immunoglobulin IgM production[1-3]. It is classified as a separate entity of the lymphoplasmacytic lymphoma type in the WHO classification[4]. Twenty-five to forty percent of patients are asymptomatic and do not need treatment, at least until disease progression, while the majority presents a wide clinical spectrum including fatigue, hyperviscosity manifestations, lymphadenopathy,organo-megaly, recurrent infections, peripheral neuropathy and other, as well as varying laboratory findings such as anemia or other cytopenias, blood lymphocytosis, hypoalbuminemia, autoimmune manifestations, increased β2-microglobulin (β2-M) and LDH, polyclonal hyper- or more frequently hypo-gammaglobulinemia, independently of IgM levels[5-8]. As a consequence of disease variability, survival ranges from 60 to 120 months. Symptomatic patients should be treated. Response to treatment is evaluated by the regression of signs and symptoms and and the decrease of monoclonal IgM serum levels; serum IgM fluctuations is used for disease monitoring[9]. Thus IgM detection, including quantification by serum protein electrophoresis, densitometry and immunofixation, is mandatory for diagnosis and monitoring of WM patients. The accuracy of the tests depends upon the position of the migrating monoclonal protein, the quantification of which requires skilled personnel[10-12]. Furthermore, user operator error may account for unexpected results particularly at low concentration.New methods for paraprotein free light and heavy chain quantification were recently developed and manufactured [13]. The serum free light chain assay (sFLC; FreeliteTM test, the Binding Site, Birmingham, UK) is a decade old by now. Using antibodies that bind exclusively to the hidden FLC molecules, it allows sensitive nephelometric quantification of circulating unbounded serum kappa and lambda light chains. sFLC measurements as well as the resultant FLC ratio (sFLCR) proved to be of clinical and prognostic utility in plasma cell dyscrasias[13-17] and also in B-cell lymphoproliferative disorders including chroniclymphocytic leukemia[18-20]. The most recent HevyliteTM immunoassay uses antibodies that target unique junctional epitopes between the heavy and light chains of each Ig molecule and determines immunoglobulin heavy chain/light chain (HLC) pairs[10,21]. The serum IgM Hevylite assay has been very recently released on the market; it specifically quantifies IgMkappa and IgMlambda separately allowing the calculation of the deriving ratios (HLCR IgMkappa/IgMlambda and vice versa)[21].Immunoglobulin (Ig) quantification by Freelite™ and Hevylite™ assays overcome some of the technical bias, while in addition they allow a much closer evaluation of the absolute value of the monoclonal intact and free component. Likewise when, for example, the monoclonal component is IgM-kappa, classical IgM measurements include the monoclonal IgM-kappa along with the remaining polyclonal IgM-kappa and the polyclonal IgM-lambda; Freelite™ measurement quantifies the monoclonal free kappa (monoclonal and polyclonal) light chain and separately the polyclonal free lambda light chain and Hevylite™ assay determines the monoclonal IgM-kappa along with the remaining polyclonal IgM-kappa while the polyclonal IgM-lambda is quantified separately.In the present study, we evaluated the contribution of the new Ig biomarkers (Freelite™ and Hevylite™) in patients with WM.
2. Materials and Methods
2.1. Patients Selection
- Seventy WM patients diagnosed and followed in 2 Hellenic centers were included in the study with their characteristics as shown in Table 1.They were 33 women and 37 men with a median age 66 years. Forty-four, 28 and 28 were in WM-IPSS[22] stage 1, 2 and 3 respectively. Anemia (Hb < 10g/L) was present in 29% of the patients, thrombocytopenia (PLT < 140x109/L) in 13%, and blood absolute lymphocytosis (Lymphocytes > 4x109/L) in 13%. Hypoalbuminaemia (serum albumin < 3.5 g/dL) was present in 22% and increased LDH (above normal) in 16%. Twenty-two percent of patients had high levels of β2-M ( ≥ 5.5 mg/L) while in 43% an extensive BMlymphoplasmacytic infiltration ( ≥ 50%) was found. Splenomegaly was present in 13% and lymphadenopathy in 17% of our patients. Paraprotein by serum immunofixation were IgM-kappa in 52 patients and IgM-lambda in 18 patients. Median IgM paraprotein level at presentation as routinely determined by nephelometry was 24.55 g/L (range 1.5-110), median IgG 8.83 g/L (range 1.96- 47.50) and median IgA 0.93 g/L (range 0.22-10.9).Fifty-one patients (73%) were or became symptomatic during follow up and received treatment according to the international criteria for treatment initiation. The 19 asymptomatic patients (27%) were only followed. Patients’ median follow-up was 47 months (range 0.5-155 months)
2.2. Sample Collection and Data Generation
- Frozen sera collected from the 70 WM patients at the time of diagnosis and from 120 blood donor healthy individuals (HI) were retrospectively analyzed using polyclonal sheep antibodies (FreeliteTM and HevyliteTM, Binding Site, UK). HLCs measurements were performed by nephelometry on a Dade Behring BNTMII nephelometer at the Binding Site laboratory in Birmingham. sFLCs measurements were also performed on a Dade Behring BNTMII nephelometer either at the Binding Site laboratory or at the Laikon’s Immunology laboratory, using FreeliteTM assay, according to the manufacturers instructions.The FLCRs were calculated with the involved sFLC as numerator. The HLCRs were calculated with the involved intact Ig as numerator and the polyclonal intact Ig of the same class as denominator, meaning that the ratio was calculated as IgM-kappa/IgM-lamda and IgM-lambda/IgM- kappa in IgM-kappa- and IgM-lambda- patients respectively.Abnormal FLCR and HLCR were defined as any value above the 95th percentile range of HI. Abnormal polyclonal sFLC was also defined using as cut-off values the 95th percentile range of the HI. For kappa-restricted patients, lambda values above 32.1 mg/L were considered as polyclonal sFLC raise and values below 12.7 mg/L as polyclonal sFLC suppression. While, for lambda-restricted patients, kappa values above 14.9 mg/L were taken as polyclonal raise and that below 1.88 mg/L as suppression. Systemic IgG and IgA hypogammaglobulinemia (IgG-SH and IgA-SH) were defined using our laboratory lower cut-off values as IgG < 7g/L and as IgA < 0.7g/L respectively. Immunoparesis of the same class (ISC) was defined as any polyclonal HLC value below the 95th percentile range of polyclonal HLCs in healthy individuals’ (HI) sera. Thus IgM-kappa patients were considered to have ISC when their IgM-lambda was below 0.17 g/L and IgM-lambda when their IgM-kappa was below 0.29 g/L . “High” sFLC, HLC and ratios were defined as any value above median. HLCs, HLCR, sFLC and FLCR values were compared with disease parameters such asbeta2-microglobulin (β2Μ), haemoglobin (Hb), serum albumin (alb), platelets (PLT) lymphocytosis, LDH, albumin (alb), bone marrow (BM) infiltration presence of splenomegaly and lymphadenopathy and survival.
2.3. Statistical Analysis
- Statistical analysis of the generated data was performed using SPSS v15.0 for windows. Hazard ratios and prognostic significance of “high” sFLC, HLC and ratios were determined by univariate Cox regression analysis. Kaplan Meier method was used for pictorial representation of survival and time to treatment. Categorical variables were compared with the chi square test (x2). P<0.05 was considered statistically significant.
2.4. Ethical Consideration
- All sera were obtained with informed consent and the use of frozen sera for the retrospective evaluation of sFLCs and HLCs was approved by the local ethical committee.
3. Results
3.1. sFLC, FLCR, HLC and HLCR in Healthy Individuals
- Serum FLCs and FLCRs, IgM-kappa, IgM-lambda and HLCRs (IgM-kappa/IgM-lambda and IgM- lambda /IgM-kappa) values are shown in Table 2.
3.2. sFLC, FLCR, HLC and HLCR in Patients
- Median involved kappa-sFLC was 45.65 mg/L (range 7.64 to 3090mg/L) and lambda-sFLC was 78.3 mg/L (range 4.49 to 1400 mg/L). Involved sFLC values above median correlated significantly with low serum albumin levels (p=0.038; p<0.05), BM infiltration ≥ 50% (p<0.001; p<0.05) and β2-M values above 3.5 mg/L or 5.5 mg/L (p=0.019; p<0.05 and p=0.035; p<0.05 respectively). Median sFLC kappa+lambda was 67.4 mg/L (range 18.44-3093.36 mg/L) and levels above median correlated significantly with anemia (p= 0.016; p<0.05), β2-M values above 3.5 mg/L or 5.5 mg/L (p=0.023; p<0.05 and p=0.002; p<0.05 respectively) BM infiltration ≥ 50% (p=0.006; p<0.05) and with advanced IPSS stage (p=0.01; p<0.05).FLCR levels were abnormal in 57/70 patients at diagnosis. Median FLCR in kappa restricted patients was 2.78 (range 0.55-9.19) and 6.95 in lambda ones (range 0.94-161.47). FLCR values above median correlated significantly with BM infiltration ≥ 50% (p=0.001; p<0.05) and anemia (p=0.016; p<0.05). In 22/70 (31%) of patients polyclonal sFLC were low and it only correlated significantly with BM infiltration ≥ 50% (p=0.029; p<0.05). Median involved IgM-kappa was 25.45 g/L (range 0.11 to 173) and IgM-lambda was 34.80 g/L (range 0.08 to 138). Involved HLC-IgM above median correlated with BM infiltration ≥50% (p=0.007; p<0.05) and with low serum albumin levels (p=0.041; p<0.05).HLCR was abnormal in 66/70 patients at diagnosis. Median HLCR in IgM-kappa WM was 185.57 (range 0.90-15000) and in IgM-lambda WM 101.9 (range 2.86-1000). HLCR values above median correlated with BM infiltration ≥ 50% (p=0.013) and the presence of splenomegaly (p=0.028). In 24/70 (34%) IgG-SH was observed, in 28/70 (40%) IgA-SH and in 27/70 (39%) ISC were observed respectively. Altogether 49/70 (70%) patients had some type of polyclonal hypogammaglobulinemia (IgG-SH, IgA-SH, ISC or polyclonal sFCL ) and it significantly correlated only to anemia (p= 0.023; p<0.05).
|
3.3. Relationship Between sFLC, HLC and Corresponding Ratios with Time to First Treatment
- Involved sFLC, sFLC (kappa+lambda) and FLCR values above median correlated significantly with time to first treatment (TFT) (p=0.002; p<0.05, p= 0.002; p<0.05 and p=0.011; p<0.05 respectively) Involved HLC and HLCR above median correlated with TFT (both p<0.001). “Figures 1A and B” showed TFT according to FLCR and HLCR. Indeed, increased total IgM paraprotein measured by standard methods was also related to TFT (p<0.001).The presence of SH, ISC sFLC polyclonal suppression or raise was not associated with TFT
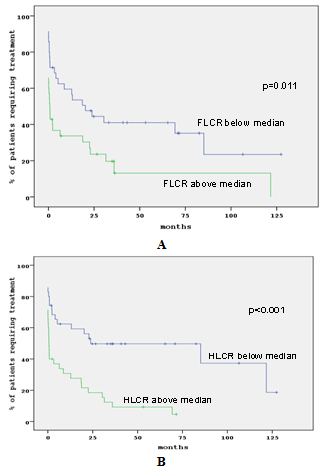 | Figure 1. TFT in correlation to F LCR (A) and HLCR (B) |
3.4. Relationship Between sFLC, HLC, Corresponding Ratios and Hypogammaglobulinemia With Overall Survival
- Disease variables with an adverse prognostic impact on survival were WM-IPSS stage (p<0.001), high β2M levels (p<0.001), abnormal LDH (p=0.001; p<0.05), the involved sFLC values above median (p=0.003; p<0.05) was shown in Figure 2A and sFLC( kappa+lambda) above median (p=0.002; p<0.05) was also shown in Figure. 2B.In addition, patients with any type ofhypogammaglobulinemia (IgG-SH, IgA-SH or ISC) had a tendency for longer overall survival (OVS) than the ones without hypogammaglobulinemia (p=0.139; p<0.05) as shown in Figure. 2CIn multivariate analysis, high β2M levels ( ≥ 5.5 mg), abnormal LDH, involved sFLC values above median kept their significance.
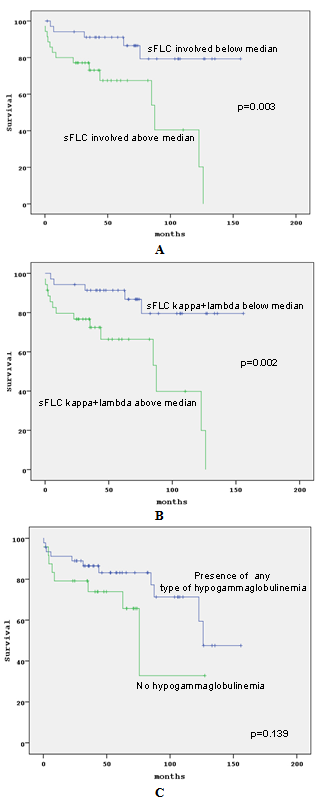 | Figure 2. OVS in correlation to sFLC (A), sFLC kappa+lambda (B) and presence of hypogammaglobulinema (C) |
4. Discussion
- Due to the WM wide range of manifestations and varying phenotype, resembling either a lymphoma or a frank plasma cell dyscrasia or even an IgM gammopathy of undetermined origin, the exact paraprotein quantification and sensitive prognostication are needed for improved diagnosis, monitoring and management[3,5]. IgM paraprotein, like any other immunoglobulin (Ig), is composed of two Ig heavy chains and two light chains. It was shown, as already mentioned, that Ig light chains are normally secreted in slight excess [10] and that the amount of the excess may consistently increase and carry prognostic information in monoclonal diseases.With regard to the contribution of sFLC levels in WM patients at diagnosis, a restricted number of studies reported that they can be increased and in such case, correlate with markers of disease activity, such as increased β2M, anemia [23,24], low serum albumin levels as well as with a shorter time to treatment[25,26]. sFLC levels were also found to constitute a marker of response and of disease progression[23]. We confirmed previous results and showed, in addition, that increased sFLC levels at diagnosis correlated significantly with a shorter overall survival. Intriguingly, sFLC levels were more valuable for monitoring and prognostication than FLCR; this was not observed in multiple myeloma[16,17] and chronic lymphocytic leukemia [27].In the present study, we also demonstrated that increased HLC-IgM correlated significantly with markers of disease activity such as extensive BM infiltration and low serum albumin levels, while high HLCR values with extensive BM infiltration also and the presence of splenomegaly. A recent study[28] suggested that, in WM, IgM secretion is related to the number of BM infiltrating plasma cells, as evaluated by CD138 staining, a finding in keep with results from our group, showing that bone marrow infiltration by CD138 expressing lymphoplasmacytes in WM strongly correlated with IgM levels[29]. In any case, although paraprotein levels are not considered reflecting tumor burden, an association with the percentage of malignant infiltrating cells has been found. Thus, it is suggested that HLC-IgM and HLCR better reflect tumor burden than IgM as classically determined. In addition, due to the wide range of clinical manifestations and varying survival of WM, the establishment of prognostic factors is needed. Retrospective studies had highlighted a number of adverse factors for survival in WM, including advanced age, anemia, thrombocytopenia, hypoalbuminemia, elevated serum β2-M, increased serum LDH, high serum IgM concentrations, a poor performance status, the pattern or the percentage of BM infiltration and the presence of lymphadenopathy and organomegaly[6-8, 30-38]. In addition, several investigators have applied and validated in WM[7, 39,40], the staging systems used in similar disorders such as IPI[41] for high grade lymphoma, ISS[42] for multiple myeloma and FLIPI[43] for follicular lymphoma. Five years ago, 7 cooperative research teams designed and successfully applied a scoring system for WM patients requiring therapy, the International Prognostic Scoring System for Waldeström's Macroglobulinemia (ISSWM)[22], that was further validated by others[44,45].In this study, the adverse prognostic impacts of increased serum β2M, LDH levels and WM-IPSS staging system were observed again, while in addition, monoclonal sFLC and.sFLC (kappa+lambda) above median correlated with a shorter overall survival. Recently, the prognostic superiority of the sum of monoclonal and polyclonal sFLC compared to the FLCR was shown in chronic lymphocytic leukemia [19,20], a disease with similarities to WM. Hence, in the present study was found that the sum also apply prognostically for TFT and OVS in WM. Polyclonal hypogammaglobulinemia is a common finding in WM; however a consistent proportion of patients present normal or even increased uninvolved Igs[7]. In the present study, we found that the presence of hypogammaglobulinemia involving Igs of the same and/or of other classes was in favour of a longer survival, while on the contrary, increased uninvolved Igs was not. This finding is in keep with the observation that in CLL, uninvolved light chain increase confer a worse outcome compared to patients with a normal secretion[19,20]. The finding is intriguing and opens issues on the biologic role of the reactive lymphocytic component in indolent lymphoproliferative disorders. Others unanswered issues on possible suppressive inherent mechanisms, such as TGFβ[46] and others that affect both normal and malignant lymphocytes and may contribute in disease control should also be explored. However, others reported on the contrary that low levels of serum IgG and IgA were associated with disease progression[47] and further investigations are needed.
5. Conclusions
- Serum FLC measurements appear useful for prognostic purposes, HLCR powerfully predicted time to first treatment and therefore may be useful in diagnostic and prognostic settings, while HLC and HLCR also help for follow-up of WM patients. In addition the Hevylite assay provides information about the amounts of the uninvolved Ig subsets that may reflect biology important mechanisms. Further studies are awaited.
References
| [1] | Lin P, Medeiros J, “Lymphoplasmacyticlymphoma/Waldenstrom’s macroglobulinemia. An evolving concept”, Adv Anat Pathol, vol.12, pp. 246-255, 2005. |
| [2] | Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, Morra E, Pangalis GA, San Miguel JF, Branagan AR, Dimopoulos MA, “Clinicopathological definition of Waldenstrom’s macroglobulinemia : Consensus panel recommendations from the second international workshop on Waldenstrom’s macroglobulinemia”, Semin Oncol., vol.30, pp.110-115, 2003. |
| [3] | Pangalis GA, Kyrtsonis MC, Kontopidou FN, Siakantaris MP, Dimopoulou MN, Vassilakopoulos TP, Tzenou T, Kokoris S, Dimitriadou E, Kalpadakis C, Tsalimalma K, Tsaftaridis P, Panayiotidis P, Angelopoulou MK, “Differential diagnosis Of Waldenstrom’s macroglobulinemia and other B-cell disorders”, Clin Lymphoma, vol.5, pp.235-240, 2005. |
| [4] | Swerdlow SH, Berger F, Pileri SA, Harris NL, Jaffe ES, Stein H. ”Lymphoplasmacytic lymphoma” In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA Stein H, Theil J, Vardiman JW., ”Who classification of tumours of haematopoetic and lymphoid tissues”, Lyon IARC Press, pp 194-195, 2008. |
| [5] | Dimopoulos MA, Kyle RA, Anagnostopoulos A, Treon SP., “Diagnosis and management of Waldenstrom'smacroglobulinemia”, J Clin Oncol., vol.23, pp.1564-1577, 2005. |
| [6] | Facon T, Brouillard M, Duhamel A, Morel P, Simon M, Jouet JP, Bauters F, Fenaux P “Prognostic factors in Waldenstrom's macroglobulinemia: a report of 167 cases”, J Clin Oncol., vol.11, pp.1553-1558, 1993. |
| [7] | Kyrtsonis MC, Vassilakopoulos TP, Angelopoulou MK, Siakantaris P, Kontopidou FN, Dimopoulou MN, Boussiotis V, Gribabis A,Konstantopoulos K, Vaiopoulos GA, Fessas P, Kittas C, Pangalis GA, “Waldenstrom's macroglobulinemia: clinical course and prognostic factors in 60 patients. Experience from a single hematology unit”, Ann Hematol., vol.80, pp.722-727, 2001 |
| [8] | García-Sanz R, Montoto S, Torrequebrada A, de Coca AG, Petit J, Sureda A, Rodríguez-García JA, Massó P, Pérez-Aliaga A,Monteagudo MD, Navarro I, Moreno G, Toledo C, Alonso A, Besses C, Besalduch J, Jarque I, Salama P, Rivas JA, Navarro B, Bladé J,Miguel JF., “Waldenstrom macroglobulinaemia: presenting features and outcome in a series with 217 cases”, Br J Haematol, vol.15, pp.575-582, 2001. |
| [9] | Treon SP, Gertz MA, Dimopoulos M, Anagnostopoulos A, Blade J, Branagan AR, Garcia-Sanz R, Johnson S, Kimby E, Leblond V,Fermand JP, Maloney DG, Merlini G, Morel P, Morra E, Nichols G, Ocio EM, Owen R, Stone MJ., “Update on treatment recommendations from the Third International Workshop on Waldenstrom's macroglobulinemia”, Blood, vol.107, pp.3442-3446, 2006. |
| [10] | Habel M-E, Emond JP “Monoclonal IgM gammopathy: Drastic bias between nephelometry and electrophoresis peak measurement” Clinical Biochemistry abstr 117, pp.1269, 2008. |
| [11] | Schnebelen A, Sweat K, Marshall J, Bornhorst J, “Alleviation of IgM monoclonal protein interference in nephelometric assays by sample treatment with reducing agent in a chaotropic salt solution”, Am J Clin Pathol., vol.137, pp.26-8, 2012. |
| [12] | Bradwell AR “Serum Free Light Chain Analysis (Plus Hevylite)”, 6th ed Birmingham, UK: The Binding Site Ltd, pp.18-58, 301-318, 2010. |
| [13] | Bradwell AR, Harding SJ, Fourrier NJ, Wallis GL, Drayson MT, Carr-Smith HD, Mead GP, “Assessment of monoclonal gammopathies by nephelometric measurements of individual immunoglobulin κ/λ ratios” , Clin Chem., vol.55, pp.1646-55, 2009. |
| [14] | Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kröger N, Einsele H, Vesole DH, Dimopoulos M, San Miguel J,Avet-Loiseau H, Hajek R, Chen WM, Anderson KC, Ludwig H, Sonneveld P, Pavlovsky S, Palumbo A, Richardson PG, Barlogie B,Greipp P, Vescio R, Turesson I, Westin J, Boccadoro M, “Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management”, Leukemia, vol.24, pp.1121-7, 2010. |
| [15] | Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT, “Serum test for assessment of patients with Bence Jones myeloma”, Lancet, vol.361(9356), pp.489-9, 2003. |
| [16] | Kyrtsonis MC, Vassilakopoulos TP, Kafasi N, Sachanas S, Tzenou T, Papadogiannis A, Galanis Z, Kalpadakis C, Dimou M, Kyriakou E, Angelopoulou MK, Dimopoulou MN, Siakantaris MP, Dimitriadou EM, Kokoris SI, Panayiotidis P, Pangalis GA, “Prognostic value of serum free light chain ratio at diagnosis in multiple myeloma”, Br J Haematol., vol.137, pp.240-3, 2007. |
| [17] | Dispenzieri A, Kyle R, Merlini G, Miguel JS, Ludwig H, Hajek R, Palumbo A, Jagannath S, Blade J, Lonial S, Dimopoulos M, Comenzo R, Einsele H, Barlogie B, Anderson K, Gertz M, Harousseau JL, Attal M, Tosi P, Sonneveld P, Boccadoro M, Morgan G, Richardson P,Sezer O, Mateos MV, Cavo M, Joshua D, Turesson I, Chen W, Shimizu K, Powles R, Rajkumar SV, Durie BG, “International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders”, Leukemia, vol.23, pp.215-24, 2009. |
| [18] | Yegin ZA, Ozkurt ZN, Yağci M, “Free light chain: a novel predictor of adverse outcome in chronic lymphocytic leukemia”, Eur J Haematol., vol.84, pp.406-11, 2010. |
| [19] | Maurer MJ, Cerhan JR, Katzmann JA, Link BK, Allmer C, Zent CS, Call TG, Rabe KG, Hanson CA, Kay NE, Slager SL, Witzig TE,Shanafelt TD, “Monoclonal and polyclonal serum free light chains and clinical outcome in chronic lymphocytic leukemia”, Blood, vol.118, pp.2821-6, 2011. |
| [20] | Morabito F, De Filippi R, Laurenti L, Zirlik K, Recchia AG, Gentile M, Morelli E, Vigna E, Gigliotti V, Calemma R, Amoroso B, Neri A,Cutrona G, Ferrarini M, Molica S, Del Poeta G, Tripodo C, Pinto A, “The cumulative amount of serum-free light chain is a strong prognosticator in chronic lymphocytic leukemia”, Blood, vol.118, pp.6353-61, 2011. |
| [21] | Keren DF, “Heavy/Light-Chain analysis of monoclonal gammopathies”, Clinical Chemistry vol. 55, pp.1606-1608, 2009. |
| [22] | Morel P, Duhamel A, Gobbi P, Dimopoulos MA, Dhodapkar MV, McCoy J, Crowley J, Ocio EM, Garcia-Sanz R, Treon SP, Leblond V,Kyle RA, Barlogie B, Merlini G, “International prognostic scoring system for Waldenstrom macroglobulinemia”, Blood, vol.113, pp.4163-70, 2009. |
| [23] | Leleu X, Koulieris E, Maltezas D, Itzykson R, Xie W, Manier S, Dulery R, Boyle E, Gauthier J, Poulain S, Tatiana T, Panayiotidis P,Bradwell AR, Harding S, Leblond V, Kyrtsonis MC, Ghobrial IM, “Novel M-component based biomarkers in Waldenstrom Macroglobulinemia (WM)”, Clin Lymphoma Myeloma Leukemia, vol.11, pp.164-7, 2011. |
| [24] | Avet-Loiseau H, Mirbahai L, Harousseau JL, “Serum immunoglobulin heavy/light chain ratios are independant risk factors for predicting progression free survival in multiple myeloma”, E.H.A, Heamatologica, vol.95 abstr 395, 2010. |
| [25] | Koulieris E, Kyrtsonis MC, Maltezas D, Tzenou T, Mirbahai L, Kafassi N, Bartzis V, Dimou M, Georgiou G, Matsouka C, Pangalis G, Panayiotidis P, Bradwell AR, Harding S, “Quantification of Serum IgM and IgM and IgM In Patients with Waldenstrom's Macroglobulinemia (WM) at Diagnosis and During Disease Course; Clinical Correlations”, ASH, Blood, vol.116, abstr.3004, 2010. In Patients with Waldenstrom's Macroglobulinemia (WM) at Diagnosis and During Disease Course; Clinical Correlations”, ASH, Blood, vol.116, abstr.3004, 2010. |
| [26] | Leleu X, Xie W, Rourke M, Banwait R, Leduc R, Roper N, Weller E, Ghobrial E, “The Role of Serum Immunoglobulin Free Light Chain In Response and Progression In Waldenstrom Macroglobulinemia”, ASH, Blood vol.116, abstr.3095, 2010. |
| [27] | Kyrtsonis MC, Vassilakopoulos T.P., Tzenou T, Kafasi N, Sachanas S, Koulieris E, Maltezas D, Dimopoulou M, Kokoris SI, Dimitriadou E, Siakantaris MP, Angelopoulou MK , Panayiotidis P, Pangalis GA, “Serum free-light chains in Waldenstrom's macroglobulinemia (WM) patient at diagnosis”, EHA, Haematologica, vol. 93(s1), abstr.1058, pp.418, 2008. |
| [28] | Itzykson R, Le Garff-Tavernier M, Katsahian S, Diemert MC, Musset L, Leblond V, “Serum-free light chain elevation is associated with a shorter time to treatment in Waldenstrom's macroglobulinemia”, EHA, Haematologica, vol. 93(s1), pp.793-4, 2008. |
| [29] | Dimitrios Maltezas, Tatiana Tzenou, Nikolitsa Kafassi, Efstathios Koulieris,Vassiliki Bartzis, Maria Dimou, Anna Efthymiou, George Georgiou, Theodoros P. Vassilakopoulos, Maria K. Angelopoulou, Panayiotis Tsaftaridis,PanayiotisPanayiotidis, Photis Beris, Marie-Christine Kyrtsonis, “Clinical Impact of Increased Serum Free Light Chains (sFLCs) or their Ratio (FLCR) in WM at diagnosis and during disease course”, Proceeding of the 6th International Workshop on Waldenstrom’s macroglobulinemia, Venice, pp. 105, 2010. |
| [30] | Pratt G, Harding S, Holder R, Fegan C, Pepper C, Oscier D, Gardiner A, Bradwell AR, Mead G, “Abnormal serum free light chain ratios are associated with poor survival and may reflect biological subgroups in patients with chronic lymphocytic leukemia”, Br J Haematol., vol.141, pp.217-2, 2009. |
| [31] | Pasricha SR, Juneja SK, Westerman DA, CameNA ,“Bone-marrow plasma cell burden correlates with IgM paraprotein concentration in Waldenström macroglobulinaemia”, J Clin Pathol, vol.64, pp.520-523, 2011 |
| [32] | Kyrtsonis MC, Levidou G, Korkolopoulou P, Koulieris E, Bartzi V, Maltezas D, Pangalis GA, Kalpadakis C, Dimou M, Georgiou G,Vassilakopoulos TP, Angelopoulou MK, Salpeas V, Tsaftaridis P, Patsouris E, Panayiotidis P, Tzenou TK., “CD138 Expression Helps Distinguishing Waldenström’s Macroglobulinemia (WM) From Splenic Marginal Zone Lymphoma(SMZL)”, Clin Lymphoma Myeloma Leukemia, vol.11, pp.99-102, 2011. |
| [33] | Morel P, Monconduit M, Jacomy D, Lenain P, Grosbois B, Bateli C, Facon T, Dervite I, Bauters F, Najman A, de Gramont A, Wattel E, “Prognostic factors in Waldenstrom's macroglobulinemia: a report on 232 patients with the description of a new scoring system and its validation on 253 other patients”, Blood, vol.96, pp.852-858, 2000. |
| [34] | Merlini G, Baldini L, Broglia C, Comelli M, Goldaniga M, Palladini G, Deliliers GL, Gobbi PG, “Prognostic factors in symptomatic Waldenstrom's macroglobulinemia”, Semin Oncol, vol.30, pp.211-215, 2003. |
| [35] | Owen RG, Barrans SL, Richards SJ, O'Connor SJ, Child JA, Parapia LA, Morgan GJ, Jack AS, “Waldenstrom macroglobulinemia: development of diagnostic criteria and identification of prognostic factors”, Am J Clin Pathol, vol.116, pp.420-428, 2001. |
| [36] | Gobbi PG, Bettini R, Montecucco C, Cavanna L, Morandi S, Pieresca C, Merlini G, Bertoloni D, Grignani G, Pozzetti U , Roberto Caporali, and Edoardo Ascari, “Study of prognosis in Waldenstrom's macroglobulinemia: a proposal for a simple binary classification with clinical and investigational utility”, Blood, vol.83, pp.2939-2945, 1994. |
| [37] | Anagnostopoulos A, Zervas K, Kyrtsonis M, Symeonidis A, Gika D, Bourantas K, Zomas A, Anagnostopoulos N, Pangalis G,Dimopoulos MA, “Prognostic value of serum beta(2)-microglobulin in patients with Waldenstrom's macroglobulinemia requiring treatment”, Clin Lymphoma & Myeloma, vol.7: pp.205-209, 2006. |
| [38] | Ghobrial IM, Fonseca R, Gertz MA, Plevak MF, Larson DR, Therneau TM, Wolf RC, Hoffmann RJ, Lust JA, Witzig TE, Lacy MQ,Dispenzieri A, Vincent Rajkumar S, Zeldenrust SR, Greipp PR, Kyle RA, “Prognostic model for disease-specific and overall mortality in newly diagnosed symptomatic patients with Waldenstrom macroglobulinaemia”, Br J Haematol, vol.133, pp.158-164, 2006. |
| [39] | Dimopoulos M, Gika D, Zervas K, Kyrtsonis M, Symeonidis A, Anagnostopoulos A, Bourantas K, Matsouka C, Pangalis GA, “The International Staging System for Multiple Myeloma is Applicable in Symptomatic Waldenstrom’s Macroglobulinemia”, Leukemia and Lymphoma, vol.45, pp.1809-13, 2004. |
| [40] | Tzenou T, Kyrtsonis MC, Kalpadakis C, Vassilakopoulos TP, Sachanas S, Galanis Z, Masouridis S, Dimopoulou M, Kokoris SI, Dimitriadou E, Korkolopoulou P, Siakantaris MP, Angelopoulou MK ,Panayiotidis P, Pangalis GA,“The follicular lymphoma International Prognostic Index is applicable in lymphoplasmacytic lymphoma / Waldenstrom’s macroglobulinemia”, EHA, Haematologica, vol.92(s1), pp.266, 2007. |
| [41] | Shipp MA, Harrington DP, Anderson JR, “A predictive model for aggressive non-Hodgkin's lymphoma”, N Engl J Med, vol.329, pp.987-994, 1993. |
| [42] | Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ,Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J, “International staging system for multiple myeloma”, J Clin Oncol, vol.23, pp.3412-3420, 2005. |
| [43] | Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero D, Coiffier B, Conde-Garcia E, Doyen C, Federico M, Fisher RI, Garcia-Conde JF, Guglielmi C, Hagenbeek A, Haïoun C, LeBlanc M, Lister AT, Lopez-Guillermo A, McLaughlin P, Milpied N, Morel P, Mounier N, Proctor SJ, Rohatiner A, Smith P, Soubeyran P, Tilly H, Vitolo U, Zinzani PL,Zucca E, Montserrat E, “Follicular lymphoma international prognostic index”, Blood, vol.104, pp.1258-1265, 2004. |
| [44] | Kastritis E, Kyrtsonis MC, Hadjiharissi E, Symeonidis A, Michalis E, Repoussis P, Tsatalas C, Michael M, Sioni A, Kartasis Z,Stefanoudaki E, Voulgarelis M, Delimpasi S, Gavriatopoulou M, Koulieris E, Gika D, Zomas A, Roussou P, Anagnostopoulos N,Economopoulos T, Terpos E, Zervas K, Dimopoulos MA, “Validation of the International Prognostic Scoring System (IPSS) for Waldenstrom's macroglobulinemia (WM) and the importance of serum lactate dehydrogenase (LDH)”, Leuk Res., vol.34, pp.1340-3, 2010. |
| [45] | Hivert B, Tamburini J, Vekhoff A, Tournilhac O, Leblond V, Morel P, “Prognostic value of the International Scoring System and response in patients with advanced Waldenström macroglobulinemia”, Haematologica, vol.96, pp.785-788, 2011. |
| [46] | Kyrtsonis M-C, Mouzaki A, Maniatis A, “Mechanisms of polyclonal hypogammaglobulinaemia in multiple myeloma (MM)”, Med Oncol, vol.16, pp.73-77, 1999. |
| [47] | Hunter ZR, Manning RJ, Hanzis C, Ciccarelli BT, Ioakimidis L, Patterson CJ, Lewicki MC, Tseng H, Gong P, Liu X, Zhou Y, Yang G,Sun J, Xu L, Sheehy P, Morra M, Treon SP, “IgA and IgG hypogammaglobulinemia in Waldenström’s macroglobulinemia”, Heamatologica, vol.95, pp.470-475, 2010. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML