-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2012; 2(6): 114-122
doi: 10.5923/j.ajmms.20120206.02
Formulation and Evaluation of Pulsatile Drug Delivery System of Metoprolol Tartarate Using Core in Cup Tablet
Prashant A. Borgaonkar 1, S. S. Bushetti 2, M Najmuddin 3
1Department of Pharmacy Acharya Nagarjuna university, Nagarjuna nagar Guntur AP and RMES’S College of Pharmacy Balaji Nagar, Gulbarga 585102-India
2Department of Industrial Pharmacy, HKES’S Matoshree Taradevi Institute of Pharmaceutical sciences, MR Medical campus, Gulbarga 585101-India
3Department of Pharmaceutics RMES’S College of Pharmacy, Balaji Nagar, Gulbarga 585102- India
Correspondence to: Prashant A. Borgaonkar , Department of Pharmacy Acharya Nagarjuna university, Nagarjuna nagar Guntur AP and RMES’S College of Pharmacy Balaji Nagar, Gulbarga 585102-India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
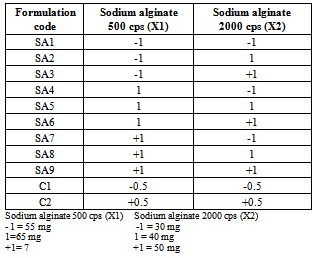
The purpose of this work is to formulate a pulsatile drug delivery system using metoprolol tartarate as model drug. A core in cup (three component tablet) is prepared where in core tablet, an impermeable material surrounding the tablet except the top and soluble hydrophilic polymer layer at the top is designed. The core tablet contains metoprolol tartarate, cellulose acetate propionate is used as impermeable membrane and sodium alginate 500 cps and sodium alginate 2000 cps used as soluble hydrophilic polymer layer .The top cover layer is prepared using 32 factorial design. Quantification of water uptake, top layer expansion, in-vitro dissolution studies, radial and axial expansion, stereomicroscopic image, and short term stability studies are performed. The concentration of top layer of hydrophilic polymer is a critical factor governing the release pattern, increase in the concentration increased lag time and delay the release .The T70% and T90 % values are also influenced by the polymer concentration with less polymer concentration of hydrophilic polymer lesser T70% and T90 % values are obtained and as the concentration increased higher values are obtained. It can be concluded from the study that pulsatile drug delivery system useful in chronotherapy of hypertension can be prepared by this technique.
Keywords: Pulsatile Release Tablet, Lag Time, Metoprolol Tartarate, Soluble Polymer
Cite this paper: Prashant A. Borgaonkar , S. S. Bushetti , M Najmuddin , "Formulation and Evaluation of Pulsatile Drug Delivery System of Metoprolol Tartarate Using Core in Cup Tablet", American Journal of Medicine and Medical Sciences, Vol. 2 No. 6, 2012, pp. 114-122. doi: 10.5923/j.ajmms.20120206.02.
Article Outline
1. Introduction
- Chronotherapy refers to treatment in which the availability of the drug to the body can be tailored to meet the therapeutic requirement. Earlier the development of the drug delivery system was based on the homeostatic theory according to this theory biological functions are constant over time however chronobiological studies contradict this theory, nearly all functions of the body including those influencing pharmacokinetic parameters display significant variation. Circadian or 24 hrs rhythm exists in blood pressure, blood flow, stroke volume, peripheral resistance, gastric acid secretion, gastro intestinal motility and gastric emptying as in[1].. Common symptoms of allergic rhinitis such as sneezing, nasal rhinorrhea, red itchy eyes were found to occur most frequently in the morning and least frequently in middle of the day as in[2].Circadian rhythm exists in arthritic pain and patients with rheumatoid arthritis experience great pain in the early morning and patient with osteoarthritis have more pain in the evening hours as in[3]. In cardiovascular diseases capillary resistance and vascular reactivity are higher in the morning and decrease latter in the day, platelet agreeability is increased leading to state of reactive hypercoagulability of the blood because of this reaction the frequency of myocardial infarction and sudden cardiac death are more prone in the morning. Ambulatory blood pressure study also con firmed the presence of circadian rhythm as in[4]. Pulsatile drug delivery system is a controlled drug delivery system where drug is released after a preprogrammed lag time. Various approaches have been used to design a pulsatile release formulation, erodible devices provided with hydrophilic polymer coating are widely used and such a system when exposed to dissolution medium undergo swelling, dissolution and/or erosion. Lag time in such a system can be controlled by using various hydrophilic polymers such as HPMC, polyethylene oxide, sodium alginate, sodium CMC. The concentration and viscosity of these polymers play a significant role in controlling the lag time and release pattern as in[5].A Capsular system in which the body part coated with impermeable polymer and hydrophilic matrix plug sealing the drug formulation is used to prepare pulsatile drug delivery system. Upon contact with aqueous fluids a rapid dissolution would occur and plug undergoes a gradual swelling and finally expluded from the body thus allowing the drug to release. The lag time in such formulation is controlled by the time needed for the plug removal and its duration depends on the physical, chemical nature, size and position of the plug. On the other hand an insoluble plug and semi permeable capsule body containing active ingredient is also used, the influx of water into capsule would lead to surge in its internal pressure ultimately resulting in plug expulsion and drug release as in[6]. Osmotic pumps are also used to achieve pulsatile release from the formulation.Metoprolol tartarate is selective beta adrenoreceptor blocking agent used to treat hypertension, angina and heart attack. The aim of the present work is to develop a simple one pulse drug delivery system based on dry coated tablet preparation. The system consist of a core tablet containing the drug, an impermeable outer shell and top cover layer barrier that dissolves at predetermined time. Cellulose acetate propionate is used as insoluble material and a blend of sodium alginate 500 cps and sodium alginate 2000 cps used as hydrophilic top layer. The hydrophilic polymers concentration is selected using 32 factorial designs to get the desired pulsatile release. The drug is released after removal of top layer of hydrophilic polymers and the lag time being adjusted by properties of the material in top cover, metoprolol tartarate is used as model drug
2. Material and Methods
- Metoprolol tartarate is received as gift sample from Torrent research centre Ahmadabad, cellulose acetate propionate is purchased from Arcos Organics New Jersey USA. Sodium alginate 500 cps is purchased from Finar Chemicals Ltd Ahmadabad and Sodium alginate 2000 cps is purchased from loba chemie Mumbai.
|
3. Evaluation
3.1. FTIR and DSC Studies
- The formulation is subjected to FTIR and DSC studies to determine the compatibility of drug and excipients.
3.2. Pre Compression Parameters
- The pre compression parameters evaluated include bulk density, tapped density, hausner’s ratio,carr’s index and angle of repose.
3.3. Post Compression Parameters
- Post compression parameters evaluated are thickness, hardness, friability, weight variation of the tablets. Further the tablets are subjected to the following evaluation parameters.
3.4. Drug Content Estimation
- Five tablets are taken and crushed, drug equivalent to 100 mg is placed in a stoppered 100 ml conical flask and drug is extracted with 25 ml of phosphate buffer PH 7.4 and filtered into 100 ml volumetric flask through Whatman No.1 filter paper (Mean pore diameter 1.5 µm) and volume is made up to 100 ml. Aliquots of the solution are filtered and analyzed for drug content by measuring the absorbance at 225 nm.
3.5. Quantification of Water Uptake
- For studying the water uptake the dissolution beakers are marked with time points 0.5, 1, 1.5 up to 6.5 hours. One tablet is placed in each of the marked dissolution beakers containing Phosphate buffer PH 7.4 maintained at 37±0.5℃ and stirred at 50 rpm. The tablets are removed from the beaker after completion of the respective time and the excess water present on the surface is removed with the help of the filter paper the tablets are weighed on shimadzu digital balance. The increase in weight of the tablet reflects the water uptake. It is estimated by equationQ=100 (Ww-Wi)/WwWhere Q = the percentage of liquid uptake and Ww and Wi are the mass of the hydrated sample and initial starting dry weight respectively.
3.6.Top Cover Layer Expansion
- The purpose of this study is to observe the top cover layer expansion and correlate with the lag time and drug release of the formulation. The tablet is attached to microscopic slide with adhesive and edges of the microscopic slides are tied with a thread and placed in a dissolution beaker (dissolution apparatus) containing 900ml of phosphate buffer PH 7.4 maintained at 37±0.5℃ and stirred at 50 rpm. At every 30 min interval the microscopic slide is withdrawn from the dissolution beaker with the help of the thread tied to the slides. The tablet is photographed using Samsung digital camera (NV 20) to study the top cover layer expansion and observe the influence of hydrophilic polymers on the lag time and release pattern.
3.7. In-Vitro Drug Release
- Dissolution studies of metoprolol tartarate is carried out in phosphate buffer PH 7.4 maintained at 37±0.5℃ using USP XXIII tablet dissolution test apparatus-II (Campbell electronics DR-6 dissolution apparatus) at 50 rpm. 5ml samples are withdrawn every 30 minutes filtered and analyzed at 225 nm using PG instruments UV-Visible spectrophotometer, equal volume of dissolution medium is replaced immediately.
3.8. Radial and Axial Measurement
- The aim of the study is to measure the radial and axial expansion because of swelling. A tablet is attached on a microscopic slide with adhesive and both ends of slides are tied with thread and further it is placed in the dissolution beaker which contained phosphate buffer PH 7.4 maintained at 37±0.5℃. The slide is removed from the dissolution beaker at every 30 min with the help of thread and increase in diameter and thickness is measured with the help of scale
3.9. Statistical Analysis
- Response surface modeling and statistical analysis are performed using (PCP disso 200 V3 software designed by Bharati vidyapeeth Poona college of Pharmacy). 3D plots and contour plots are constructed using the same software
3.10. Stereomicroscopic Studies
- The prepared tablet contains three component insoluble polymer layer at the bottom and surrounding the core tablet, core tablet at the centre and hydrophilic swellable polymer at the top. To study the inner arrangement of the assembly the tablet is cut vertically at the centre and photographed using stereomicroscope.
3.11. Stability Studies
- Short term stability studies are performed at a temp of 40±2℃ and 75±5 % RH over a period of three months(90 days).The tablets of the best formulation are placed in stability chamber(Thermal instruments and equipments) and samples are withdrawn every month visually examined and evaluated for drug content and in-vitro dissolution studies.
4. Results and Discussion
4.1. FTIR and DSC Studies
- In the present study the drug used is metoprolol tartarate, since the drug contain tartaric acid salt it gives a broad absorption peak around 3450 cm-1 corresponding to hydroxyl group of the drug as well as hydroxyl group of tartaric acid residue. It also contains aromatic C-C-H and aliphatic C-C-H which is indicated by the absorption peak at 2980 cm-1 and 2872 cm-1. The N-H absorption peak of the drug as merged with the hump is observed at 3150 cm-1, the IR of this compound also exhibited peak at 1690 cm-1 and 1632 cm-1 corresponding to the carboxylic acid residue of tartaric acid. All the data obtained from the IR spectrum are in agreement with the known structure of the metoprolol tartarate. The above drug is subjected to formulation with different excipients. In cellulose acetate propionate a weak absorption is noticed at 3497 cm-1 probably because of free hydroxyl group present in the moiety. The C-C-H of the type which is part of the ring structure of the sugar or C-C-H of the alkyl residue attached to the hydroxyl group are noticed at 2982 cm-1 and 2944 cm-1 respectively. The ester moiety of acetate as well as propionate have exhibited strong absorption peak at 1743 cm-1 which is the normal place of absorption of carboxyl esters.Sodium alginate contains number of hydroxyl group of primary and secondary nature and hence IR spectrum of this compound gave a broad hump at 3400 cm-1 where in primary as well as secondary hydroxyl group absorption peak have been merged to give rise to a hump. It is noticed that only aliphatic C-C-H absorption peak are noticed in the IR spectrum suggesting that it has no aromatic residue. The sodium salt of carboxylic acid residue present in the molecule has also exhibited a broad peak at 1610 cm-1 these are the characteristics peak noticed for sodium alginateA core in cup pulsatile drug delivery system of metoprolol tartarate is formulated and the formulation obtained is taken for IR measurement. The formulation contains metoprolol tartarate, cellulose acetate propionate and sodium alginate, the IR spectrum of the formulation exhibited a weak absorption peak at 3480 cm-1 and a peak at 3150 cm-1 due to the O-H and N-H of the drug moiety. The presence of C-H due to the aromatic C-H and aliphatic C-H observed at 2980 cm-1 and 2872 cm-1, the strong peak are observed at 1739 cm-1 and 1632 cm-1 due to the ester group of the cellulose acetate propionate. The data obtained suggests that all the characteristic functionalities of the drug and the excipients have remained unchanged during the process of formulation, confirming that no chemical reaction has taken place. The drug metoprolol tartarate is subjected to DSC measurements in which it starts melting at 121.23℃giving rise to melting peak at 124.26℃ this is because pure drug is not used its salt is used and because of this broad melting range is observed but not the Sharpe.The formulation is subjected to DSC studies, the melting range is obtained at 123.14℃ and 66.58℃ thereby suggesting that drug and excipients have given rise to melting range 123.14℃ and 66.58℃. The reduction in melting range of the drug by 1℃ is due to the preparation of physical mixture during the formulation. This strongly suggests that drug has remained intact and not under gone any chemical change during the formulation processA core in cup tablet for Pulsatile release of metoprolol tartarate is prepared. A total of 9 formulations are prepared by keeping the concentration of drug and impermeable layer constant, soluble hydrophilic polymer concentration is selected by using 32 factorial design.
4.2. Pre Compression Parameters
- The bulk density is in the range of 0.64 g/cc to 0.68 g/cc and tapped density in the range of 0.75g/cc and 0.79 g/cc, carr’s index in the range of 12.80 % to 15.18 %, hausner’s ratio in the range of 1.14 to 1.17 and angle of repose in the range of 24.18 to 26.24 degree as shown in Table 2. It can be concluded from the study that formulation has good flow property and can be compressed into a tablet which meets all the requirements.
4.3. Post Compression Parameters
- The appearance of the tablets is smooth and uniform, hardness of the tablets is in the range of 5.5 to7.0 kg/cm2, thickness in the range of 5.5 to 6.5 mm, weight variation in the range of 3 to 4 % and drug content in the range of 98.40 to 99.82 %.The prepared pulsatile drug delivery system has three parts a core tablet, impermeable layer and soluble hydrophilic top layer consisting of sodium alginate 500 cps and sodium alginate 2000 cps used in 32 factorial design .The top hydrophilic layer in the formulation is designed to absorb water and swell when it gets in contact with liquid, after swelling a gel layer is formed around the tablet which acts as a barrier that prevents the liquid from reaching the core surface. In total 9 formulations are prepared and the tablet with less amount of polymer on top layer achieved less swelling when compared to tablets having higher concentration of polymer on top layer. Visual observation shows a greater swelling for SA9 formulation with polymer ration (+1,+1). Thus from the study it is observed that the top cover layer swelling and lag time are influenced by polymer concentration and viscosities.
 | Figure 1. Quantification of water uptake studies of SA9 formulation |
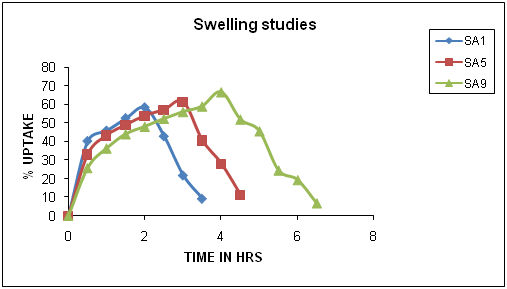 | Figure 2. Quantification of water uptake studies of SA9 formulation |
4.5. Top Layer Expansion Study
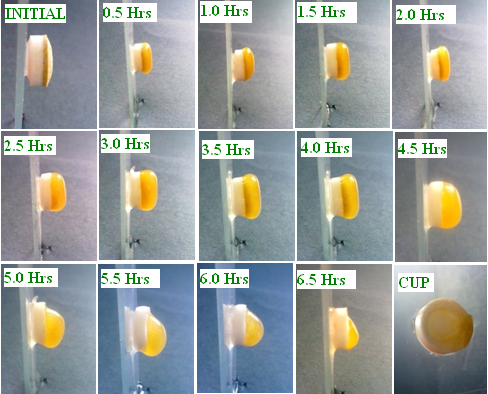 | Figure 3. Top cover layer expansion studies of formulation SA9 |
4.6. In-vitro Drug Release
- In-vitro dissolution studies of all the formulation confirmed a typical pulsatile release as shown in Figure 4 with a initial lag time (no release period) is observed and as the concentration of the polymer used in top layer increased the lag time is also increased leading to decreased release of the drug. The lag time observed in formulation SA1, SA2, SA3 with polymer concentration of sodium alginate 500 cps and sodium alginate 2000 cps (-1,-1), (-1,1) and (-1,+1) are 2.5 hrs, 3 hrs and 3 hrs, formulation SA4, SA5, SA6 with polymer concentration of (1,-1), (1,1) and (1,+1) gave a lag time of 3.5 hrs,4.0 hrs and 4 hrs, formulation SA7,SA8, SA9 with polymer concentration of (+1,-1),(+1,1) and (+1,+1) gave lag time of 4.5 hrs,4.5 hrs an 5 hrs respectively. Influence of polymer concentration on drug release was similar to that of lag time, with increase in polymer concentration the release was prolonged. In formulation SA1 (-1,-1) 99.19 % drug is released in 5 hrs, SA2 (-1,1) 98.99 % drug released in 5.5 hrs, SA3 (-1,+1) 99.18 % drug released in 6 hrs, SA4 ( 1,-1) 99 % drug released in 7hrs, SA5 (1,1) 98.49 % drug released in7.5 hrs SA6 (1,+1) 99.31 % drug released in 8 hrs, SA7 (+1,-1) 99.63 % drug released in 8.5 hrs, SA 8 (+1,1) 99.14 % drug released in 9.5 hrs and finally formulation SA9 (+1,+1) 99.14 % drug released in 10.5 hrs.
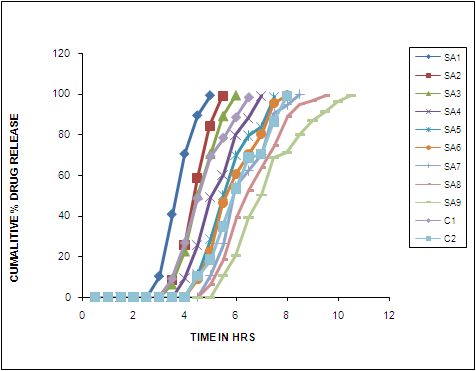 | Figure 4. In-Vitro Release Profile of Formulation SA1 TO SA9 |
|
 | Figure 5. Comparison of dissolution parameters (T70 % and T90 %) |
4.7. Radial and Axial Expansion Measurement
- SA1, SA5 and SA9 formulations are selected to study radial and axial expansion due to swelling three formulation contained low, medium and high concentration of polymer on top layer.In SA1 the initial diameter is 9 mm and maximum radial expansion of 10 mm (1.1 fold) is observed at 1 hr time interval followed by a gradual decrease, in SA5 the initial diameter is 9.5mm and maximum radial expansion of 11.5 mm(1.2 fold) is observed at 2.0 hrs and in SA9 the initial diameter is 9 mm and maximum radial expansion of 12.5 mm (1.4 fold) is observed at 2.5 hrs.The axial (thickness) expansion is also measured in SA1 the initial thickness is 5.5 mm and maximum axial expansion of 12 mm (2.1 fold) is observed at 2.0 hrs, in SA5 the initial thickness is 6.0 mm and maximum axial expansion of 15.5mm (2.6 fold) is observed at 3.0 hrs and in case of SA9 the initial thickness is 6.0 mm and maximum axial expansion of 17.5mm (2.9 fold) is observed at 4.0 hrs respectively. The study confirms the preferential axial expansion, the polymer swells more in axial direction compared to radial direction.
4.8. Statistical Analysis
- In order to investigate the factors systematically and to arrive at an optimum formulation a 32 factorial design is employed in the present investigation. A polynomial equation is derived for the lag time and T 70% (time required for 70% of drug release) using PCP disso 2000 V3 software (developed by Bharati vidyapeeth’s Poona college of Pharmacy). The studied factors(independent variable) are concentration of sodium alginate 500 cps, sodium alginate 2000cps and the response ( dependent variable) studied are lag time and T 70%.A second order polynomial equation that fitted the data is as follows Y=b0 + b1 X1+ b2 X2 + b X1 X2+ b11 X12+ b22 X22 Where Y is the independent variable bo is the arithmetic mean response of nine batches, b1,b2, b11 b12 and b22 are coefficient computed from the observed experimental values X1 and X2 stand for main effect and the polynomial terms X12 and X22 are included to investigate the non linearity.The reduced model with significant values for both the factors areLag time Y1 = 3.777 + 0.916 + 0.250T 70% Y2 = 5.818 + 1.461 + 0.513Positive sign in front of the term indicate synergistic effect while negative sign indicate antagonist effect of the factor as in[10]. The positive sign observed in the present study indicated synergistic effect and as the concentration of polymer increase the lag time is increased and the time required for T 70% drug release is also is increased, the p value less than 0.05 observed indicant the significance effect.
4.9. Response Surface Analysis
- The quadratic models generated by regression analysis are used to construct 3D response surface plots. A linear synergistic effect between two independent variable on response is observed. Figure 6/ Figure 8. The increase in lag time and T70 % with increase in polymer concentration can be attributed to the greater swelling of the polymer with increased concentration.Figure7/Figure9.Two extra formulation C1 and C2 are designed as check points and evaluated for lag time and T 70 % .The predicted lag time are 3.239 hours and 4.315 and the observed lag time are 3.0 hours and 4.0 hours respectively. Similarly the predicted T70% value for C1 and C2 are 4.832 hours and 6.804 hours and the observed values are 4.911 and 6.986 hours respectively. The nearness of predicted and observed values indicates the validity of the derived equation for dependent variable.
 | Figure 6. Response surface plots showing the influence of polymer con centration on lag time |
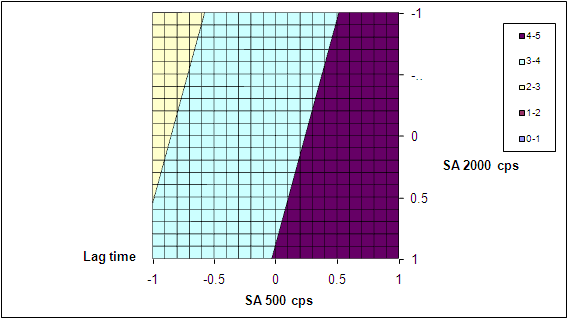 | Figure 7. Contour plots showing the influence of polymer concentration on lag time |
 | Figure 8. Response surface plots showing the influence of polymer concentration on T 70% ( Time required for 70% of drug release) |
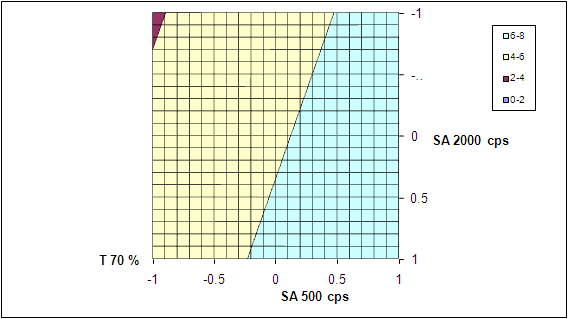 | Figure 9. Contour plots showing the influence of polymer concentra tion on T 70% (Time required for 70% of drug release) |
4.10. Stereomicroscopic Studies
 | Figure 10. Stereomicroscopic images of SA9 formulation |
4.11. Drug Release Kinetics
|
4.12. Stability Studies
- The formulation SA9 is subjected to short time stability studies for 3 months ( 90 days) at 40±2℃ and 75±5 % RH . Samples are withdrawn every month and analyzed for drug content and invitro release. The drug content and dissolution data of the stability studies does not show any significant variation as shown in table 5/Figure 11.
|
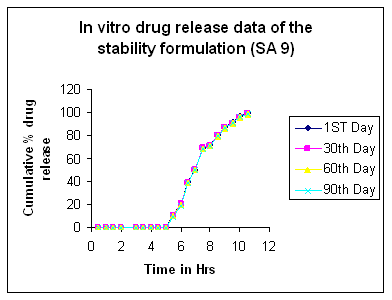 | Figure 11. In-Vitro drug release studies of formulation SA |
5. Conclusions
- Core In cup pulsatile drug delivery system of metoprolol tartarate is formulated to increase the therapeutic effectiveness of the drug. The formulation contains three components a core table, impermeable layer and soluble hydrophilic polymer layer. The in-vitro dissolution studies indicates drug release after lag time and the lag time is controlled by the soluble hydrophilic polymer concentration and its properties. The lag time is increased by increasing the concentration of polymer. The formulation SA9 with (+1,+1) concentration of sodium alginate 500 cps and sodium alginate 2000 cps gave a lag time of 5hrs and 99.14% of drug release in 10.5 hrs. Thus the drug release from the tablet can be tailored to meet the therapeutic requirement of the patients.The formulation with greater concentration of soluble hydrophilic polymer swells to a greater extent and hence the swelling rate is directly proportional to the polymer concentration. Formulation SA9 shows a greater swelling compared to other formulation.FTIR and DSC studies confirm that the drug and polymer are compatible with each other. Short term stability studies indicate no significant changes in drug content and dissolution rates.Thus with this system a pulsatile drug delivery system can be prepared to increase the therapeutic effectiveness of the drug.
ACKNOWLEDGEMENTS
- The authors are thankful to Torrent research centre Ahmadabad, Gujarat-India for providing the drug sample, the authors are also thankful to rmes College of pharmacy, Gulbarga-India, for providing the facilities to carry out the research work.
References
| [1] | Bjorn L, “Relevance for chronopharmacology in practical Medicine”, Seminar in peronatology, 24(4), pp.280-290, 2000. |
| [2] | Smolensky MH,Lemmer B et al, “Chronobiology and chronotherapeutics of allergic rhinitis and bronchial asthma American j .Respi.crit care med”, 59, pp.852-882, 2007. |
| [3] | Bruguerolle B, Labrecque G et al “Rhythmic pattern in pain and their chronotherapy”, Advanced drug delivery reviews, 59, pp.883-895, 2007. |
| [4] | Bjorn L, “The importance of circadian rhythms on drug response in hypertension and coronary heart diseases”, Pharmacology and therapeutics, 111, pp.629-6512006. |
| [5] | Efenkatis M,Koligliati S et al,” Design and evaluation of a dry coated drug delivery system, an impermeable cup, swellable top layer and pulsatile release”, International journal of Pharmaceutics, 311, pp-147-156, .14 |
| [6] | Mastiholomath VS, Dandagi PM et al, “International journal of pharmaceutics”, 328 pp. 49-56, 2007. |
| [7] | Roy P, Shahiwala A,” Multiparticulate formulation approach to pulsatile drug delivery current perspective”, Journal of controlled release”,134 pp.74-80, 2009. |
| [8] | Bussemer T,Bodmeier RA,” Drug delivery: Pulsatile encyclopedia of pharmaceutical technology”, pp-1287-1297, 2006. |
| [9] | Sharma S, Pawar A,” Low density multiparticulate system for pulsatile of Meloxicam”, International journal of Pharmaceutics, 313, pp.150-158, 2006. |
| [10] | Romacn CH,Ayala DE et al, “Chronotherapy of hypertension: administration time dependent effects of treatment on circadian pattern of blood pressure”, Advance drug delivery reviews, 59, pp.923-939, 2007. |
| [11] | Karvasa E ,Georgarakis E et al,” Felodipine nanodispersion as active core for predictable pulsatile chronotherapeutics using using PVP/HPMC blends as coating layer”, International Journal of Pharmaceutics,313, pp.189-197, 2006. |
| [12] | Krogel I, Bodemeier R,” Floating or pulsatile drug delivery system based on coated effervescent core”, International journal of Pharmaceutics, 187, pp.175-184, 1999. |
| [13] | Lin HL, Lin SY,” Release characteristics and invitro-invivo correlation of pulsatile pattern for a pulsatile drug delivery system activated by membrane rupture via osmotic pressure and swelling”, European journal of pharmaceutics and biopharmaceutics, 70, pp.289-301, 2008. |
| [14] | Avachat A,Kotwal V, “Design and evaluation of matrix based controlled release tablets of Diclofenac sodium and Chondroitin sulphate”, AAPS Pharmascitech, pp.8(4):E1-E6, 2007. |
| [15] | Li B, Zhenget al, “A novel system for three pulse drug based on tablet in capsule device. International journal of pharmaceutics”, 352, pp.156-164, 2008. |
| [16] | Karvasa E,Georgarakis E et al, “Application of pulsatile miscible blends with enhanced mucoadhision properties for adjusting drug release in predictable pulsatile chronotherapy”, European journal of pharmaceutics and biopharmaceutics, 64, pp.115-126, 2006. |
| [17] | Maroni A , Idenuma R, “Design of rate and time programming drug release device using a hydrogel: pulsatile drug release from K-carrageeana hydrogel device by surface erosion of hydrogel”, Colloids and surfaces,20, pp.355-359, 2001. |
| [18] | Maroni A,ZemaL, “Oral pulsatile delivery: rational and chronopharmaceutical formulaion.International journal of Pharmaceutics”, 398, pp.1-8, 2006. |
| [19] | Roy P, “Shahiwala A.Stastical optimization of ranitidine HCl floating pulsatile delivery system for chronotherapy of nocturnal acid breakthrough,” European journal of pharmaceutical sciences, 37, pp.363,2009. |
| [20] | Saigal N, Baboota S et al, “Site specific chronotherapeutics drug delivery system,” A patent review recent patents on drug delivery and formulation, 3, pp.64-70, 2009. |
| [21] | Bussemer T, Bodmeier R, “Formulation parameters affecting the performance of coated gelatin capsules with pulsatle release profiles,” International journal of Pharmaceutics, 267, pp.59-68, 2003. |
| [22] | Howard NE, Wilson Cg et al, “Evaluation of Pulsincap to provide regional delivery of Dofetilide to the human GI tract, ” International journal of Pharmaceutics, 236, pp.27-34, 2007. |
| [23] | Sawada T,Konda H et al, “Time release compression coated core tablet containing Nifidipime forchronopharmacotherapy,” International journal or Pharmaceutics, 280, pp.103-111, 2004. |
| [24] | Jiang HL, Zhu Kj, “Pulsatile protein release from a laminated device comprising of polyanhydrides and PH sensitive complexes”, 194, pp.51-60 2004. |
| [25] | Maggi L,Conte U et al, “Press coated tablets for the sequential pulse administration of two different drugs,” 99, pp.173-179, 1993. |
| [26] | Fan TY,Wei SL et al, “An investigation of pulsatile release tablets with ethylcellulose and eudragit L as film coating material and cross linked polyvinylpyrrolidone in the core tablet,” Journal of controlled release, 77, pp.245-251, 1993. |
| [27] | Coughan DC,Quilty FP, “Effect of drug physicochemical properties on swelling deswelling kinetics and pulsatile drug release from thermoresponsive poly(N-isopylaacrylamide) hydrogels. Journal of controlled release”, 98, pp.97-114, 2004. |
| [28] | Sungthongieen S,Puttipipakhachorn S et al,” Development of pulsatile release tablets with swelling and rupturable layer’” Journal of controlled release,147, pp.147-159, 2004. |
| [29] | Sher P, Ingavale G et al , “low density porous carrier based conceptual drug delivery system. Microporous and mesoporous material”, 102, pp.290-298, 2007. |
| [30] | Mcconvill JT,Rose Ac et al,”The effect of wet granulation on the erosion behavior of an HPMC lactose table, used as a rate controlling component in pulsatile drug delivery capsule formulation”, European journal of Pharmaceutics and biophrmaceutics, 57, pp.541-549, 2007. |
| [31] | Schellekens RCA,Stellard F et al.,”Pulsatile drug delivery to ileo-colonic segments by structured incorporation of disintegrants in PH responsive polymer coating”, Journal of controlled release, 932, pp.91-98, 2007. |
| [32] | Bussemer T,Peppas NA et al, “Evaluation of the swelling hydration and rupturing properties of the swelling layer of the rupturable pulsatile drug delivery system”, European journal of pharmaceutics and biopharmaceutics, 56, pp.261-270, 2003. |
| [33] | Dashevsky A,Mohamad, “Development of pulsatile multiparticulate drug delivery system coated with aqeous dispersion aquacoat ECD”, International journal of Pharmaceutics,318, pp.124-131, 2003. |
| [34] | Bussemer T,Dashevsky A et al, “A pulsatile drug delivery system based on rupturable coated heard gelatin capsules. Journal of controlled release”, 93, pp.313-339, 2003. |
| [35] | Mohamad A, Dashevsky, “PH independent pulsatile drug delivery system based on hard gelatin capsules coated with aqeous dispersion aquacoat ECD.European journal of pharmaceutics and biopharmaceutics”, 64, pp.173-179, 2006. |
| [36] | Giunchedi P,magi L, “Ketoprofen pulsatile absorption from multiple unit hydrophilic matrices”, International journal of pharmaceutics,77,pp.177-81, 1991. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML