-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2012; 2(3): 50-58
doi: 10.5923/j.ajmms.20120203.05
Alleviating Effect of Phyllanthus niruri on Sensorimotor and Cognitive Changes Induced by Subacute Chlorpyrifos Exposure in Wistar Rats
Suleiman F. Ambali 1, 2, Annas O. Makinde 2, Muftau Shittu 2, Stephen A. Adeniyi 2, Favour O. Mowuogwu 3
1Department of Veterinary Physiology and Pharmacology, University of Ilorin, Ilorin, Nigeria
2Department of Veterinary Pharmacology
3Department of Biochemistry, Ahmadu Bello University, Zaria, Nigeria
Correspondence to: Suleiman F. Ambali , Department of Veterinary Physiology and Pharmacology, University of Ilorin, Ilorin, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Objective: Studies in animal models and humans have shown that chlorpyrifos (CPF) causes sensorimotor and cognitive changes. Apart from inhibiting acetylcholinesterase (AChE), the induction of oxidative stress is one of the other molecular mechanisms implicated in OP-evoked toxicity. The present study evaluated the alleviating effect of Phyllanthus niruri, a flavonoid rich plant, on subacute CPF-evoked sensorimotor and cognitive changes in Wistar rats. Design: Thirty five adult male Wistar rats divided into five groups of seven rats were used for the study. Groups 1 and II were given soya oil (2 ml/kg) and CPF (10.6 mg/kg~1/8th of LD50), respectively. Group III was administered methanol extract of Phyllanthus niruri (MEP) at 500 mg/kg only. Group IV was administered MEP (250 mg/kg) and CPF (10.6 mg/kg), 30 min later while group V was administered with MEP (500 mg/kg) and CPF (10.6 mg/kg), 30 min later. The regimens were administered by gavage once daily for 28 days during which periodic evaluation of sensorimotor and cognitive performances were carried out. Thereafter, the brain was evaluated for AChE activity and malonaldehyde (MDA) concentration. Results: CPF caused deficits in sensorimotor reflexes and cognition, increased brain MDA concentration and decreased AChE activity, which were all mitigated by MEPN. Conclusion: The study concludes that mitigation of sensorimotor and cognitive deficits elicited by CPF was mitigated by MEP partly due to the antilipoperoxidative and acetylcholinesterase restoration properties of its flavonoid constituent.
Keywords: Chlorpyrifos, Sensorimotor, Cognition, Brain lipoperoxidation, Amelioration, Phyllanthus niruri
Article Outline
1. Introduction
- There has been increasing concern about the pervasive use of pesticides to combat the menace posed by pest to humans and animals. Organophosphate (OP) compounds are one of the most widely used accounting for about 50% of the global insecticide use[1]. OP compounds form a large family of over 50,000 chemical agents with biological properties that have important and sometimes unique implications for man [2]. There is some evidence that long-term low-level OP exposure may produce adverse neurological effects, including neurobehavioral changes and impaired peripheral nerve function[3-5]. In addition, long-term exposure to OP compound may increase the risk of some neurodegenerative diseases such as Parkinson’s disease[6] and Alzheimer disease [7]. Most of the ill-health following exposure to OPs has been attributed to inhibition of acetylcholinesterase (AChE), whose function is to degrade the neurotransmitter acetylcholine (ACh)[8]. However, recent studies have confirmed that even exposures that are obviously asymptomatic at or below the threshold for inhibition of cholinesterase result in neurobehavioral abnormalities in animal models[9-11] and in humans[12-17]. Because of the impairment of functions that occurs at a dose below that which inhibit AChE activity, current literature has justifiably implicated other mechanisms[18,19] including oxidative stress in both acute and chronic OP poisoning[20].Chlorpyrifos (CPF) is a broad spectrum chlorinated OP insecticide used extensively in a wide variety of formulations to control agricultural and other pestiferous insects[21]. It was first marketed for use in 1965 and was subsequently used widely to control variety of pest such as termites, fleas, cockroaches, mosquitoes[22]. The widespread use of CPF and its accidental or careless application have resulted in the death of many species of both target and non-target organisms[23,24]. CPF has received wide attention as is the most studied OP insecticide[9-11]. Studies have shown neurobehavioral deficit following CPF exposure in animal models[25-28]. Phyllanthus niruri, commonly known as chanca piedra belongs to the family Euphorbiaceae, indigenous to subtropical and tropical areas throughout the world including [29]. It has been used in herbal medicine worldwide for centuries where it grows[30], and has been employed for treatment of different ailments[31]. P. niruri has been shown to contain polyphenolic flavonoid. The flavonoid component of P. niruri has been identified as a strong antioxidant, and hence could ameliorate oxidative stress[32,33]. A direct relationship between antioxidant activity and phenolic content of plant extract has been reported[34,35]. The phenolic compounds are an important group of secondary metabolites, which are synthesized by plants because of plant adaptation to biotic and a biotic stress condition such as infection, water stress, and cold stress[36]. Studies have shown that many flavonoids are more potent antioxidant than vitamins C and E[37,38] and may therefore be viewed as promising therapeutic agents for free radical pathologies[39]. We have earlier shown the mitigating effect of vitamins C[26,27] and E[28] on neurobehavioral changes evoked by CPF in rats. Furthermore, methanolic extract of P. niruri has been shown in our previous study to mitigate hemotoxicity evoked by CPF in rats[40]. The present study was therefore aimed at evaluating the alleviating potential of the methanolic extract of P. niruri on subacute CPF-induced sensorimotor and cognitive changes in Wistar rats.
2. Materials and Methods
2.1. Materials
2.1.1. Experimental Animals
- Thirty five male Wistar rats (12-14 weeks old) weighing 115-126g used for this study were obtained from the Animal House of the Department of Veterinary Physiology and Pharmacology, Ahmadu Bello University, Zaria. The rats were housed in steel cages and fed standard rat chow while water was provided ad libitum.
2.1.2. Plant Collection, Identification and Preparations
- Phyllanthus niruri was collected from . It was taxonomically identified and authenticated in the Herbarium of the Department of Biological Science, , , with voucher No 2522. The plant was air dried under shade and then powdered using pestle and mortar. The powdered material was extracted with methanol at a ratio of 1:5 w/v in a glass funnel for 24 h, with periodic shaking to enhance the extraction process. Thereafter, the solution was filtered and the filtrate concentrated in-vacuo using the rotary evaporator coupled to a thermocirculator. The residue labeled methanolic extract of P. niruri (MEP) was further air-dried to a constant weight and then preserved in a refrigerator at 4℃ until required for use.
2.1.3. Chemical Acquisition and Preparations
- Commercial grade CPF (20% EC) marketed as Termicot® (Sabero Organics, Gujarat limited, ) was prepared by reconstituting in soya oil (Grand Cereals and Oil Mills Ltd., ) to make 10% stock solution. The extract was reconstituted in distilled water to 10% stock solution. All other chemicals used in the study were of analytical grades and were obtained from Sigma Inc, .
2.2. Methods
2.2.1. Animal Treatments
- The thirty five (35) Wistar rats were divided at random into 5 groups of seven (7) animals each. Group I (S/oil), the control group was administered soya oil (2ml/kg), which was the vehicle of administration of the chemical and the extract while group II (CPF) was administered reconstituted CPF only (10.6 mg/kg). Group III (MEP) was administered MEP only (500 mg/kg) and soya oil (2ml/kg) while group IV (LMEP+CPF) was administered MEP (250 mg/kg (Ambali et al., 2010a) followed by reconstituted CPF (10.6 mg/kg) 30 min later. Group V (HMEP+CPF) was administered MEP (500 mg/kg) followed by reconstituted CPF (10.6 mg/kg) 30 min later[40]. The regimens were given once daily by gavage for a period of 28 days. The periodic evaluation of neurobehavioral parameters measuring motor and neuromuscular coordination, motor strength, and learning and short-term memory were carried out. The neurobehavioural parameters were assessed by two raters blinded to the experiment.
2.2.2. Evaluation of Motor Coordination
- Motor coordination was assessed using the performance beam walk apparatus as described by Ambali et al.[26]. Briefly, individual rat from each group was allowed to walk across a wooden black beam of 106-cm length, beginning at 17.2-cm width and ending at 1.0-cm width. Periodic widths were marked on the side of the apparatus. On each side of the narrowing beam, there was a 1.8-cm step-down to a 3.0-cm area where subjects may step if necessary. As the subject walked across, the width of the beam at which they stepped down was recorded by one rater on each side, and this was repeated twice during each session. This procedure was carried out on days 0, 14 and 28.
2.2.3. Evaluation of Neuromuscular Coordination
- Neuromuscular coordination was assessed by evaluating the performance on incline plane as described by Ambali et al. [26]. Briefly, each rat was placed on an apparatus made with an angled rough wooden plank with thick foam pad at its bottom end. The plank was first raised to an inclination of 35°, and thereafter gradually increased stepwise by 5° until the subject could no longer stay and be situated horizontally on the plank for 3s, without sliding down. Angles were measured and marked on the apparatus beforehand, and were obtained by propping the plank on a vertical bar with several notches. The test was performed with the head of the rat first facing left and then right hand side of the experimenter. The highest angle at which each rat stayed and stood horizontally, and facing each direction was recorded. Two trials were performed for each testing period. This procedure was carried out on each animal from all the groups on days 0, 14 and 28 of the study.
2.2.4. Evaluation of Treatments on Learning Acquisition
- The effect of CPF on learning task in rats and the possible ameliorative effect of MEP were assessed 48 hours to the termination of the test using the step-down inhibitory avoidance learning task as described by Zhu et al.[41]. The apparatus used for the learning test was an acrylic chamber 40 x 25 x 25 cm consisting of a floor made of parallel 2-mm-caliber stainless steel bars spaced 1 cm apart. An electric shock was delivered through the floor bars. A 2.5-cm-high, 8 x 25 cm wooden platform was placed on the left extreme of the chamber. Each animal was gently placed on the platform. Upon stepping down, the rat immediately received a single 80-volt foot shock. If the animal did not return to the platform, the foot shock was repeated every 5s. A rat was considered to have learned the avoidance task if it remained on the platform for more than 2 min. The number of foot shocks was recorded as an index of learning acquisition.
2.2.5. Assessment of the Effect of Treatments on Short-Term Memory
- Short-term memory was assessed in individual rat from each group using the step-down avoidance inhibitory task as described by Zhu et al.[41] twenty four hours after the assessment of learning. The apparatus used for the memory test was the same used earlier for the assessment of learning. In this test, the rat was again placed gently on the platform 24 hours after performing the learning task. The time an animal remained on the platform was recorded as an index of memory retention. Staying on the platform for 2 min was counted as maximum memory retention (ceiling response).
2.2.6. Effect of Treatments on Brain Lipoperoxidation
- The level of thiobarbituric acid reactive substance, malonaldehyde (MDA) as an index of lipid peroxidation was evaluated on the brain samples using the method of Draper and Hadley[42] as modified[43]. The principle of the method was based on spectrophotometric measurement of the color developed during reaction of thiobarbituric acid (TBA) with MDA. The MDA concentration in each sample was calculated by the absorbance coefficient of MDA-TBA complex 1.56 x 105 cm/M and expressed as nmol/mg of tissue protein. The concentration of protein in the brain homogenates was evaluated using the method of Lowry et al.[44].
2.2.7. Evaluation of the Effect of Treatments on Brain Acetylcholinesterase Activity
- Acetylcholinesterase activity was evaluated using the method of Ellman et al.[45] with acetylthiocholine iodide as a substrate. Briefly, the whole brain sample of each animal was homogenized in a cold (0–4℃) 20 mM phosphate buffer saline (PBS) incubated with 0.01 M 5,5-dithio-bis(2- nitrobenzoic acid) in 0.1 M PBS, pH 7.0. Incubations were allowed to proceed at room temperature for 10 min. Then, acetylthiocholine iodide (0.075 M in 0.1 M PBS, pH 8.0) was added to each tube, and absorbance at 412 nm was measured continuously for 30 min using a UV spectrophotometer (T80+ UV/VIS spectrometer, PG Instruments Ltd., Liicestershire, LE 175BE, United Kingdom) with slit width of 1 cm. AChE activity expressed as IU min/mg protein was calculated based on the rate of color change per minute.
2.3. Statistical Analysis
- Data obtained from neurobehavioral parameters measured repeatedly expressed as mean + SEM were subjected to repeated measure analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Data obtained from neurobehavioral parameters measured once in the study and those obtained from biochemical parameters were analyzed using one-way analysis of variance followed by Tukey’s test using Graphpad prism version 4.0 for Window. Values of P < 0.05 were considered significant.
3. Results
3.1. Effect of Treatments on Motor Coordination
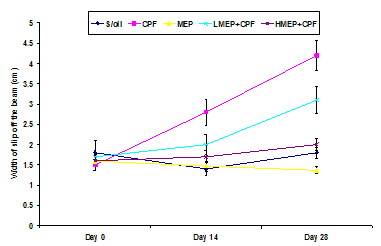
- The dynamics of the effect of treatments on cumulative mean beam walk is shown in Figure 1. There were no significant changes (P>0.05) in the beam walk when the days of evaluation were compared with each other in the S/oil, MEP and HMEP+CPF groups. However, there was a significant decrease (P<0.05) in the width at which the CPF group slipped off the beam on day 14 when compared to that recorded on day 0 and day 28, respectively. There was a significant decrease (P<0.01) in the width of slip off the beam at day 0 compared to day 28. The width of slip in the LEP+CPF group was significantly higher (P<0.01) on day 28 compared to those of day 0 (P<0.01) or day 14 (P<0.05) but no significant change at day 14 versus day 0.
3.2. Effect of Treatments on Neuromuscular Coordination
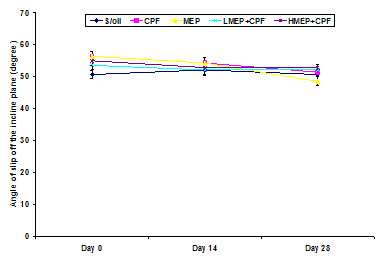
- There were no significant changes (P>0.05) in the incline plane performance in between the days of assessment in the S/oil, MEP, LMEP or HMEP group. There was a significant decline in the angle of slip off the incline plane in the CPF group on day 28 when compared to that recorded on day 0 (P<0.01) or day 14 (P<0.05).There were no significant changes (P>0.05) in the incline plane performance in between the groups on days 0 and 14. However, there was a significant decline (P<0.05) in incline plane performance on day 28 in the CPF group when compared to that of MEP or HMEP group (Figure 2).
3.3. Effect of Treatments on Grip Time
- There were no significant changes (P>0.05) in the grip time recorded in between the days of evaluation in the S/oil, MEP and HMEP+CPF groups. However, there was a significant decline (P<0.01) in the grip time recorded on day 28 compared to those of days 0 or 14 in the CPF group. There was a significant decline (P<0.05) in the grip time of CPF group on day 14 compared to that of day 0. There was a significant decrease (P<0.01) in the grip time in the LMEP+CPF group on day 28 relative to that of day 0, but no significant change (P>0.05) in the value recorded on day 14 compared to those of day 0 or day 28.There was no significant change (P>0.05) in the grip time in between the groups on day 0. However, the grip time of CPF group was significantly lower (P<0.05) compared to that of MEP or HMEP+CPF group on day 14. Although not significant (P>0.05), the grip time of CPF group on day 14 was relatively lower compared to that of S/oil (13%) or LMEP+CPF (6%) group. The grip time of CPF group on day 28 was significantly lower compared to that of S/oil (P<0.01), MEP (P<0.01), LMEP+CPF (P<0.05) and HMEP+CPF (P<0.01) groups, respectively. There was a significant decline in the grip time recorded on day 28 in LMEP+CPF group relative to that of S/oil (P<0.05) or MEP (P<0.01) group (Figure 3).
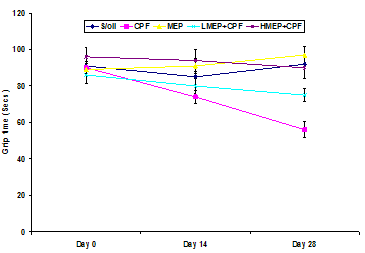 | Figure 3. Effect of soya oil (S/oil), chlorpyrifos (CPF), methanolic extract of Phyllanthus niruri (MEP), 250 mg/kg MEP+chlorpyrifos (LMEP+CPF) and 500 mg/kg +chlorpyrifos (HMEP+CPF) on grip time |
3.4. Effect of Treatment on Learning Acquisition
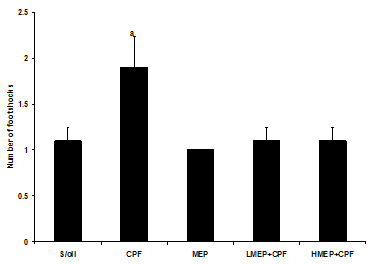
- There was a significant increase (P<0.05) in the number of footshock applied to CPF group compared to that of MEP group. Although not significant (P>0.05), the mean number of footshocks applied to the CPF group was 42% higher that of the S/oil, LMEP+CPF or HMEP+CPF group (Figure 4).
3.5. Effect of Treatments on Short-Term Memory
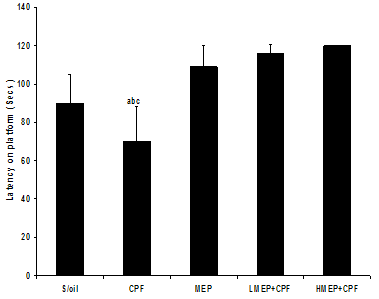
- There was a significant decline (P<0.01) in latency on the platform in the CPF group compared to that of S/oil, MEP, LMEP+CPF and HMEP+CPF groups, respectively. Although not significant (P>0.05), the mean duration of stay on the platform in the HMEP+CPF group was relatively longer (2.5%) than that of LMEP+CPF group (Figure 5).
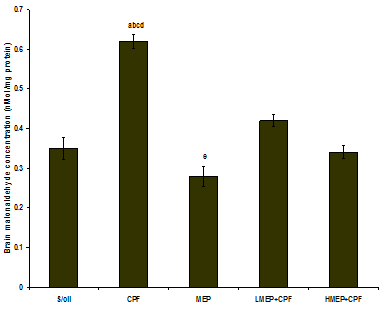
3.6. Effect of Treatments on Brain Lipoperoxidation
- There was a significant increase (P<0.01) in the MDA concentration in the CPF group compared to that recorded in the S/oil, MEP, LMEP+CPF and HMEP+CPF groups, respectively. There was a significant increase (P<0.01) in MDA concentration in the LMEP+CPF group relative to that recorded in MEP group (Figure 6).
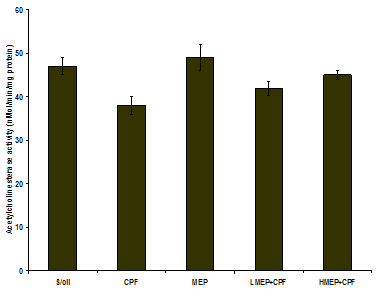
3.7. Effect of Treatments on Brain Acetylcholinesterase Activity
- There was a significant decrease in brain AChE activity in the CPF group relative to that recorded in S/oil (P<0.05) and MEP group (P<0.01). Although not significant (P>0.05), the mean brain AChE activity in the CPF group was relatively decreased when compared to that of LMEP+CPF (9.5%) or HMEP+CPF (15.5%) group. The mean brain AChE activity in the HMEP+CPF group increased by 7% over that recorded in the LMEP+CPF group (Figure 7).
4. Discussion
- The increased brain MDA concentration engendered by CPF indicates lipoperoxidative changes hence oxidative damage in the organ. This finding agrees with those from previous studies[25-27,46]. The brain is vulnerable to oxidative stress due to its biochemical and physiological properties[47]. Apart from harboring large amount of oxygen in a relatively small mass, the brain contains a significant quantity of metals (Fe), and has fewer antioxidant mechanisms than other tissues[48,49]. ROS can directly attack the polyunsaturated fatty acids of the cell membranes and induce lipid peroxidation. MDA is the end-product of lipid peroxidation, which is a process where reactive oxygen species (ROS) degrade polyunsaturated fatty acids. This compound is a reactive aldehyde and is one of the many reactive electrophile species that cause toxic stress in cells and form advanced glycation end-products. The production of this aldehyde is used as a biomarker to measure the level of oxidative stress in an organism[50].The significant reduction in brain MDA concentration hence lipid peroxidation engendered by the plant extract is related to its antioxidant property. The antioxidant activity of flavonoids results from their ability to donate a hydrogen atom from an aromatic hydroxyl group to a free radical, and yield a resonance-stabilized phenolic radical[51]. In addition, the amphiphilicity of the flavonoids enhances their ability to trap chain-initiating radicals at the interface of the membranes, preventing progression of the radical chain reaction[51,52]. They also enhance the activity of the endogenous antioxidants, chelate transition metals that plays catalytic role in free radical generation and inhibit the propagation of lipoxygenase reaction[50,52]. The inhibition of AChE activity by CPF in the present study is a reflection of its anticholinesterase effect. Apart from the direct inhibition of AChE through occupation of the esteratic site of the enzyme, oxidative stress has been indirectly linked with AChE inhibition. AChE is one of the membrane bound enzymes and lipoperoxidation of the membrane has been shown to alter its activity[54,55]. The attenuation of AChE inhibition by the plant extract may be related to its antioxidant effect which results in reduced membrane lipoperoxidation hence alteration of AChE activity. Several antioxidant molecules such as vitamins C and E have been shown restore AChE activity[26-28].The deficit in motor coordination in the CPF group could be attributed to CPF-induced damage to the cerebral and cerebellar cortices, probably as a result of apoptosis- a toxic endpoint of CPF neurotoxicity in the brain[56] apparently due to induction of oxidative stress. The brain is highly susceptible to oxidative stress-induced injury because of its relatively low antioxidant capacity[57] and high amount of peroxidable fats and significant quantity of trace metal oxidant (Fe)[48,49]. However, this effect was ameliorated in a dose-dependent manner by the MEP, which contains flavonoids, an antioxidant polyphenolic compound[26]. This resulted in the restoration of the CPF-induced oxidative damage to the brain. Furthermore, MEP may have facilitated recovery from damage to the locus coeruleus (LC), the norepinephrine-source in the brain[58]. Norepinephrine plays an important role in the locomotor deficit caused by cortical injury[59]. Norepinephrine facilitates the recovery from locomotor deficit by alleviating injury-induced decrease and turnover of norepinephrine levels in the cortex[60,61]. The preservation of the cellular integrity of LC by MEP may have aided the improvement in the performance of the beam-walk test. The deficit in neuromuscular coordination characterized by poor inclined plane performance observed in rats exposed to CPF only agreed with the findings of Ambali et al.[26]. This could be attributed to CPF-induced oxidative stress causing damage to the cerebral cortex, and subsequent neuronal cell death from delayed apoptosis[58,62]. However, this effect was ameliorated by MEP in a dose-dependent manner and may be partly ascribed to the antioxidant capacity of flavonoids contents of the extract in combating the CPF-evoked oxidative stress. The effect of CPF on cognitive deficits could partly be attributed to oxidative stress produced by it[26-28], leading compromization of the cellular integrity and function of the brain neurons. This finding is consistent with OP compound-induced alterations in cognitive deficits such as impaired learning acquisition and short-term memory observed in previous studies[26,63-65]. The impaired AChE activity by CPF recorded in the present study may have contributed to the cognitive decline. It is believed that OP compounds play a role in memory loss by producing cholinergic dysfunction at the level of the synapse[66]. Overstimulation followed by depression of cholinergic receptors and downregulation of muscarinic receptors[67] may have contributed to the memory dysfunction evoked by CPF. Furthermore, many studies have linked central cholinergic system to synaptic plasticity, learning and memory processes[68,69]. In addition, De Groot et al.[70] stated that accumulated ACh resulting from AChE inhibition by OP compounds leads to activation of glutamatergic neurons and the release of the excitatory L-glutamate amino acid neurotransmitter. This in turn produces increased depolarization and subsequent activation of the N-Methyl-D-aspartate (NMDA) subtype of glutamate receptors and the opening of NMDA ion channels resulting in massive Ca2+ fluxes into the post-synaptic cell leading to neuronal degeneration that involves free radical generation which results in oxidative stress[71]. The ability of CPF to induce cytotoxicity directly on the hippocampal cells via the induction of apoptosis, irrespective of its effect on AChE[65] may have contributed to the memory deficits observed in rats exposed to CPF only. However, the CPF-induced cognitive deficits have been shown by the present study to be ameliorated significantly by the MEP in a dose-dependent manner. This may be attributed to the polyphenolic flavonoids present in MEP, which provided protection against the damaging effects of reactive oxygen species[72], either through the prevention of cell death after glutamate injury by scavenging radicals, maintaining the correct glutathione levels or inhibiting Ca2+ influx, which represents the last step in the cell death cascade[73].
5. Conclusions
- The present study has demonstrated the ability of MEP to mitigate the sensorimotor and cognitive deficit engendered by subacute CPF exposure partly due to the anti- lipoperoxidative and AChE restoration properties due to its flavonoid constituent. Apart from these, the non antioxidant related activity of flavonoid such as its role in signaling and promotion of PON 1 gene may have aided in the mitigation of the CPF-evoked neurobehavioral deficit.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML