-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2012; 2(3): 41-44
doi: 10.5923/j.ajmms.20120203.03
Effect of Ondansetron alone or in Combination with Dehydrobenzperidole and Dexamethasone on Discharge Time Following Gynecological Laparoscopy
Hanne Ulka Petersen , Claus Hemmingsen
Department of Anaesthesiology, Frederiksberg Hospital, Copenhagen, Denmark
Correspondence to: Claus Hemmingsen , Department of Anaesthesiology, Frederiksberg Hospital, Copenhagen, Denmark.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
We aimed to examine a possible accelerated discharge time after gynecological laparoscopy when treated with a combination of ondansetron, dehydrobenzperidole and dexamethasone as compared to ondansetron alone. Sixty ASA 1-2 women were included in this prospective double-blind study and were randomised to either peroperative ondansetron 4 mg, dehydrobenzperidole 0.625 mg and dexamethasone 8 mg or to ondansetron 4 mg only. General anaesthesia was induced by fentanyl, propofol, mivacurium and maintained by sevoflurane in 50% oxygen. Patients were tracheal intubated and had a orogastric tube during the laparoscopy. The neuromuscular block was allowed to wear of without the use of neostigmine. In case of postoperative nausea and vomiting in the ondansetron only group, escape medication with dehydrobenzperidole and dexamethasone was given. The discharge criteria at the day surgery unit were fulfilled at 7h 45min (range 3h 10min - 24h 16min) after the end of surgery in the women treated with ondansetron only, but was accelerated to 5h 11min (range 3h 9min – 21h 8min) in the women having peroperative treatment with both ondansetron, dehydrobenzperidole and dexamethasone (p<0.01). Postoperative nausea and vomiting was evident in 4% at arrival at day surgery recovery after treatment with the triple combination, but in 16% in the patients treated with ondansetron alone (p<0.01). In conclusion, peroperative treatment with a combination of ondansetron, dehydrobenzperidole and dexamethasone allows a significantly accelerated discharge time after gynecological laparoscopy and causes a significantly reduced incidence of nausea and vomiting.
Keywords: Gynecological Laparoscopy, Discharge Time, Postoperative, Nausea and Vomiting, Ondansetron, Dehydrobenzperidole, Dexamethsone
1. Introduction
- Postoperative nausea and vomiting (PONV) are common complications after gynecological laparoscopy and in the absence of prophylactic antiemetics the incidence has been described up to 75% even with the use of modern anesthetics. Numerous studies have shown that the administration of prophylactic antiemetics, either alone or in combination, can reduce this incidence significantly 1.In a study of patient attitudes regarding surgery, vomiting, nausea, and pain were ranked as three of the primary outcomes that patients found equally least desirable 2.Because of cost-saving potential and the convenience for the patients, gynecological laparoscopy is routinely performed in day surgery. PONV however, remains a major problem and has been found to reduce patient satisfaction in this setting. Since the causes of PONV are multifactorial, with at least four different neurotransmitter systems implicated in the etiology, no single antiemetic drug possesses the ability to prevent PONV in all patient populations 1. The specific delay in discharge time caused by PONV after gynaecological laparoscopy and its dependency upon various anti-emetic regimes remains unclear 3.Aim of the present study was therefore to examine a possible accelerated discharge time after gynecological laparoscopy when treating patients with a combination of ondansetron, dehydrobenzperidole and dexamethasone as compared to ondansetron alone. Our theoretical null hypothesis was that there was no difference in discharge time depending upon mono or triple anti-emetic medication.
2. Methods
- Sixty ASA 1-2 female patients, ages 18 – 70 years, were enrolled in this prospective double-blind study and were randomised to either peroperative ondansetron 4 mg, dehydrobenzperidole 0.625 mg and dexamethasone 8 mg or to ondansetron 4 mg only. The study was performed in the elective day surgery center at Frederiksberg Hospital, Copenhagen. The protocol was approved by The Danish Medicines Agency and by the local Ethics Committee, all patients received oral and written information, and gave informed consent prior to participation.Patients experiencing wonted nausea and vomiting, having a BMI>35, scored to ASA-group 3 or higher, being pregnant or lactating, being planned for admission, or being allergic to the medications used were excluded from participating in the study.General anaesthesia was induced by fentanyl, propofol, mivacurium and maintained by sevoflurane in 50% oxygen. Patients were tracheal intubated and had a orogastric tube during the laparoscopy. The neuromuscular block was monitored by Train-of-Four stimulation and was allowed to wear off without the use of neostigmine. Postoperative pain was treated with a standard regime of oxycodone and ibuprofen. The anti-emetic medication was administered intravenously in a double-blinded fashion immediately after the induction of anesthesia. The personnel at the awakening, at the recovery room and at day surgery were blinded to the anti-emetic used. The intravenous fluid administration consisted of 1000 ml of isotonic saline.The level of nausea and vomiting was scored immediately after awakening in the operating theatre and then every 15 minutes in the recovery room and in day surgery. Nausea was scored by using the scoring shown in table 1, and in case of any level of postoperative nausea and vomiting in the ondansetron only group, escape medication with dehydrobenzperidole 0.625 mg and dexamethasone 8 mg was administered. Pain after arrival at recovery or day surgery was treated with oxycodon 2.5 – 10 mg.Home readiness in the day surgery was evaluated at 15-minutes intervals by using a postanesthesia scoring system shown in table 2, the maximum score being required for discharge, and time to discharge ready was recorded for all patients.
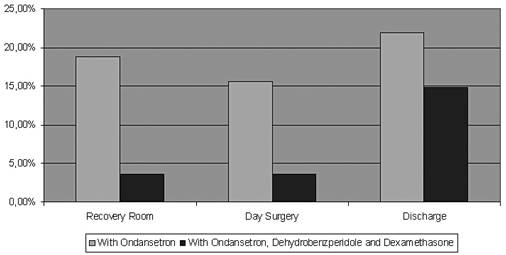 | Figure 1. Incidence of nausea and vomiting |
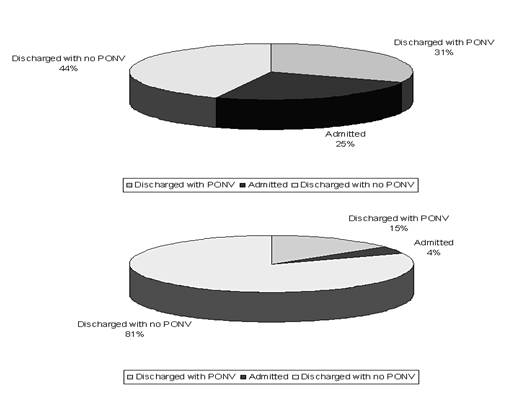 | Figure 2. Status at discharge |
3. Results
- The discharge criteria at the day surgery unit were fulfilled at 451 min (interquartile rage 244,411) after the end of surgery in the women treated with ondansetron only, but was accelerated to 319 min (217,309) in the women having peroperative treatment with both ondansetron, dehydrobenzperidole and dexamethasone (p<0.05)(Table 3 and Fig 1). The two groups spent an equal amount of time in the recovery room (97 vs. 90 min), whereas patients receiving monotherapy spent 361 min (139,337) in the day surgery compared to 218 min (136,220) for the triple-therapy group (p<0.05)All patients presenting PONV in the ondansetron only group (56%) were supplied with dehydrobenzperidole and dexamethasone.Postoperative nausea and vomiting was evident in 4% at arrival at day surgery recovery after treatment with the triple combination, but in 16% in the patients treated with ondansetron alone (p<0.01) (Fig 2).At the time of discharge from hospital, 31% in the ondansetron only group had light PONV, and 25% were admitted for further observation at the surgical ward. In the triple treatment group, 15% had light PONV (p<0.05) and 4% were admitted for the surgical ward (p<0.05).
4. Discussion
- Despite numerous publications and guidelines, PONV is still the most common reason for poor patient satisfaction in the postoperative period. The effect of various regimes against PONV has been thoroughly described in several previous studies. The present study adds information on the effect on discharge time in a day surgery unit with rigid criteria of discharge. Our data shows that treatment with a combination of ondansetron, dehydrobenzperidole and dexamethasone accellerates discharge after gynecological laparoscopy in addition to reducing the incidence of PONV.Further, our data shows that the triple treatment minimizes the frequency of patients needing a discharge time longer than 400 min. This reduces the amount of patients needing admission to a surgical ward due to their recovery exceeding opening hours of day surgery from 25 to 4%.
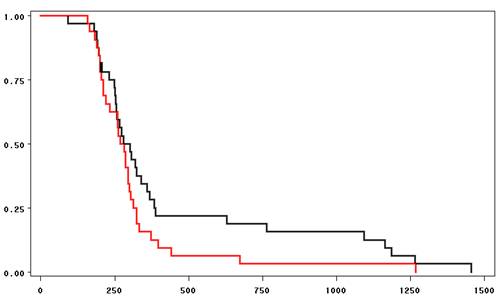 | Figure 3. Kaplan-Meier survival curves showing time of discharge (minutes) in Ondansetron alone (black) and combination group (red) |
References
| [1] | Kovac AL: Prevention an d treatment of postoperative nausea and vomiting. Drugs 2000;59:213-243 |
| [2] | Macario A, Weinger M, Carney S, Kim A: Which clinical anesthesia outcomes are important to avoid? Anesth Analg 1999;89:652-658 |
| [3] | White PF, O´Hara JF, Roberson CR, Wender RH, Candiotti KA: The impact of current antiemetic practices on patient outcomes: A prospective study on high-risk patients. Anest Analg 2008;107:452-458 |
| [4] | Chan MT, Choi KC, Gin T, Chui PT, Short TG, Yuen PM, Poon AH, Apfel CC, Gan TJ. The additive interaction between ondansetron and droperidol for preventing postoperative nausea and vomiting. Anesth Analg 2006;103:1155-1162 |
| [5] | Tramer MR. A rational approach to the control of postoperative nausea and vomiting: Evidence from systematic reviews. Part 2, Recommendations for prevention and treatment, and research agenda. Acta Anaesthesiol Scand 2001;45:14-19 |
| [6] | Gan TJ, White PF, Scuderi PE, Watcha MF, Kovac A. FDA "black box" warning regarding use of droperidol for postoperative nausea and vomiting: is it justified?. Anesthesiology. 2002;97:287 |
| [7] | Horowitz BZ, Bizovi K, Moreno R. Droperidol--behind the black box warning. Acad Emerg Med. 2002;9:615-8 |
| [8] | Habib AS, Gan TJ. Pro: The Food and Drug Administration Black box warning on droperidol is not justified. Anesth Analg. 2008;106:1414-7 |
| [9] | Ludwin DB, Shafer SL. Con: The black box warning on droperidol should not be removed (but should be clarified!). Anesth Analg. 2008;106(5):1418-20 |
| [10] | Rappaport BA. FDA response to droperidol black box warning editorials. Anesth Analg. 2008;106(5):1585 |
| [11] | Leslie JB, Gan TJ. Meta-analysis of the safety of 5-HT3 antagonists with dexamethasone or droperidol for prevention of PONV. Ann Pharmacother. 2006;40(5):856-72 |
| [12] | Ho CM, Ho ST, Wang JJ, Tsai SK, Chai CY. Dexamethasone has a central antiemetic mechanism in decebrated rats. Anesth Analg 2004;99:734-9 |
| [13] | Tramer MR, Reynolds JM, Moore RA, McQuay HJ. Efficacy,dose-response and safety of ondansetron in prevention of postoperative nausea and vomiting. A quantitative systematic review of randomized placebo-controlled trials. Anesthesiology1997;87:1277–89 |
| [14] | Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg 2000;90:186 –94 |
| [15] | Dupeyron JP, Conseiller C, Levarlet M, Hemmingsen C, Schoeffler P, Pedersen FM, Gribomont B, Kaplan LA. The effect of oral ondansetron in the prevention of postoperative nausea and vomiting after major gynaecological surgery performed under general anaesthesia. Anaesthesia 1993;48:214-218 |
| [16] | Liu K, Hsu C-C, Chia Y-Y. The effective dose of dexamethasone for antiemesis after major gynecological surgery. Anesth Analg 1999;89:1316 –18 |
| [17] | Tang J, Wang B, White PF, et al. The effect of timing of ondansetron administration on its efficacy, cost-effectiveness and cost benefit as a prophylactic antiemetic in the ambulatory setting. Anesth Analg 1998;86:274–82 ondansetron administration on its efficacy, cost-effectiveness and cost benefit as a prophylactic antiemetic in the ambulatory setting. Anesth Analg 1998;86:274–82 |
| [18] | Wang J-J, Ho S-T, Tzeng J-I, Tang S-S. The effect of timing of dexamethasone administration on its efficacy as a prophylactic antiemetic for postoperative nausea and vomiting. Anesth Analg 2000;91:136 –9 |
| [19] | Gan TJ, Meyer T, Apfel CC, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg 2003;97:62–71 |
| [20] | Coloma M, Duffy LL, White PF, et al. Dexamethasone facilitates discharge after outpatient anorectal surgery. Anesth Analg2001;92:85–8 |
| [21] | Apfel CC, Laara E, Koivuranta M. A simplified risk score for predicting postoperative nausea and vomiting. Conclusions and cross-validations between two centers. Anesthesiology 1999;91:693–700 |
| [22] | Paech MJ, Rucklidge M, Lain J, Dodd PH, Bennett E, Doherty DA. Ondansetron and dexamethasone dose combinations for prophylaxis against postoperative nausea and vomiting. Anesth Analg 2007;104:808–14 |
| [23] | White H, Black H, Jones M, Marfan GC. Randomized comparison of two anti-emetic strategies in high-risk patients undergoing day-case gynaecological surgery. British Journal of Anaesthesia 2007;98:470-6 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML