-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2012; 2(3): 29-35
doi: 10.5923/j.ajmms.20120203.01
Co-Morbid Factors Related to Surgical Complications in Kidney Transplant Patients
Maroun M. Abou-Jaoude 1, 2, 3, Haidar Nasser 4, Alain N. Khalaf 1, Walid J. Abou-Jaoude 1, 2, 3, Ziad Daoud 3
1Transplant unit, Middle East Institute of Health, Beirut, Lebanon
2Transplant unit, St-Georges University Medical Center, Beirut, Lebanon
3Faculty of Medicine and Medical Sciences, University of Balamand, Beirut, Lebanon
4Faculty of Medicine, Lebanese University, Lebanon
Correspondence to: Ziad Daoud , Faculty of Medicine and Medical Sciences, University of Balamand, Beirut, Lebanon.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract We have studied retrospectively the demographics and different post transplantation morbidities associated with surgical complications in 200 kidney transplant recipients between May 1997 and January 2008. Patients were divided into 2 groups: Group I including 177 patients without surgical complications and Group II including 23 patients who had surgical complications. Baseline demographics and later co-morbidities were analyzed. The baseline characteristics between the 2 groups did not differ significantly, including donor and recipient age and sex, recipient’s body mass index, cause of original renal disease, transplantation date, dialysis duration, recipient’s degree of sensitization and pre-transplantation diabetes. However significant difference between the 2 groups included: pre and post-transplant hemoglobin blood level differences (2.6 ± 1.8 mg/dl in Group I versus 4.1 ± 2.0 mg/dl in Group II), number of post-transplant transfusions (0.4 ± 0.8 in Group I versus 2.2 ± 3.7 in Group II), duration of hospital stay (10.9 ± 4.3 days in Group I, versus 17.5 ± 9.2 days in Group II), mean serum creatinine upon discharge (1.47 ± 0.84 mg/dl in Group I versus 2.7 ± 2.87 mg/dl in Group II), death and graft failure at 6 months post-transplant (2 in Group I versus 2 in Group II and 3 in Group I versus 5 in Group II respectively). We conclude that surgical complications were associated with significant short and long term co-morbidities, including duration of hospital stay, serum creatinine upon discharge, and death and graft failures at 6 months post-transplantation.
Keywords: Transplantation, Kidney, Surgical Complications
1. Introduction
- Although kidney transplantation (KT) is described as the best treatment modality for patients with end stage renal disease (ESRD)[1] and in spite of the advanced and improved diagnostic interventions, surgical complications remain a great concern and a significant clinical issue that increases morbidity and costs and potentially leads to graft loss after KT[2,3].Despite the improvements in graft and patient survival achieved in the previous two decades[4,5] mainly those related to the decreased incidence of acute rejection[6], the occurrence of some surgical complications such as lymphoceles[7] and wound infections[8] remain a major concern after KT. The reasons for this increase do not necessarily imply a surgery related problem, since several risk factors are associated, including the donor age[9,10] and the state of the recipient health[11]. The use of more potent and newer immunosuppressant regimens have contributed to the decrease of rejection rate[6], however, some reports show the strong correlation between some of these drugs and the incidence of surgical complications in KT[7,8]. Vascular complications represented 1-2% of postoperative complications, including renal artery thrombosis, renal artery stenosis and renal vein thrombosis with spontaneous graft ruptures[12]. Urological complications were reported in a range between 2.6 and 15% in some large cohort studies[13]; ureteral leaks and stenosis being the most frequent[4]. Many retrospective studies have addressed the risk factors and reported the incidence for surgical complications in KT[14]. However, their impact on the post-operative course of kidney transplant recipients was rarely reported. In this study, we analyzed retrospectively post-transplant surgical complications in our patients with the risk factors and the impact on morbidity and mortality up to 6 months after KT.
2. Material and Methods
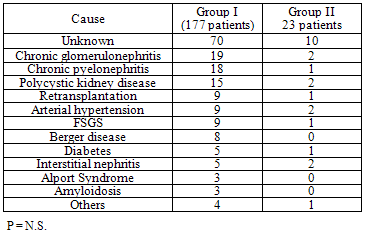
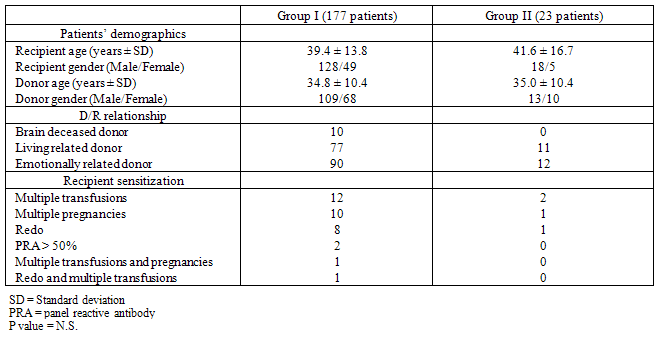
- Patients and Donors: Between May 1997 and January 2008, 200 adult patients (146 males and 54 females; mean age 39.6 ± 14.1 years) were operated of KT at and The Middle East Institute of Health in . Of these, 191 were first transplants, and the others having a second re-transplant. The donor age (34.9 ± 10.3 years) and gender distribution (122 males and 78 females) were comparable to those of patients, and donors types comprised living-related (n = 88), emotionally related (n = 102), and 10 brain-dead donors. Sensitization consisted of 9 re-transplants, 14 multiple transfusions, 11 multiple pregnancies, 2 pre-transplant panel- reactive antibody score (PRA) > 50%, 1 multiple pregnancies and transfusions and 1 re-transplant and multiple transfusions. While chronic glomerulonephritis and pyelonephritis were the most common, the cause of renal disease was not clear in 80 patients because of late diagnosis (Table 1). The pre-transplant dialysis duration ranged from 0 to 124 months (mean 14.6 ± 19.8 months). Twenty six patients had a preemptive KT.Operation: All transplants were heterotopic inserted in the iliac fosse. Vascular anastomoses were done with the recipient external iliac vessels in an end-to-side manner, the vein first then the artery using 2 continuous prolene 5-0 for the vein and 1 continuous prolene 6-0 on one side and separated stitches on the other side for the artery. Vesico -ureteral anastomosis was done using the Lich-Gregoir technique as described previously[12]. To minimize urological complications, an internal double-J ureteric stent was inserted before ending the uretero- neocystostomy, and then removed 6 weeks after KT by cystoscopy[13]. A closed drain was left in the operative area before wound closure, and removed when the drainage is <50 ml/day. Continuous low dose heparin (10 000 - 15 000 units/day) IV infusion was given routinely to our recipients starting 6 hours after the transplant procedure and continued till the patient’s discharge from the hospital. Thereafter, oral baby aspirin and pravastatin were given continuously. Immunosuppression regimen: Maintenance of immunosuppression was similar between the 2 groups (P = N.S.) and consisted of intravenous methylprednisolone (500 mg), given during surgery than tapered progressively over the next four weeks to 0.2 mg/kg/day than to 5 mg/day of prednisone (Pred). Cyclosporine microemulsion (CyA-me) was given after the transplant (5 mg/kg bid), or was delayed in case of slow graft function (SGF) or delayed graft function (DGF); the dose was adjusted to a C2 levels of 1700 ng/ml during the first month. In some patients, Tacrolimus (Tac) was given in place of CyA-me, at a dose of 0.1 mg/kg bid, and monitored for a trough level of 12-15 ng/ml during the first month. Mycophenolate mofetil (MMF) was started 48 hours before KT at 1 gm bid (in CyA-me patients) or 500 mg tid (in Tac patients). Diagnosis of surgical complications: Surgical complications were all symptomatic and the diagnosis was suspected clinically then confirmed by radiology or laboratory tests. Vascular complications were diagnosed using ultrasound (), nuclear imaging, magnetic resonance angiography or angio CT-Scan. Diagnosis of urological complications was made by US and confirmed using per-cutaneous anterograde pyelography for ureteral stenosis or laboratory analysis of the drain liquid in case of ureteral leak. Cultures were made to all fluids collected from the drain or the wound.Statistical Analysis: The data were analyzed using SPSS version 13.0 (SPSS Inc., ). Data are reported as the mean ± SD or percent of total. Inter-group significance was determined by Student t-test (continuous variables) and Fisher’s exact test (categorical variables). Statistical significance set at P < 0.05.
|
3. Results
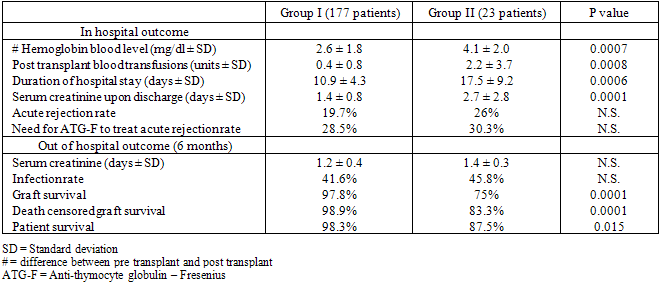
- Surgical complications were diagnosed in 23 patients (Group II) and comprised: surgical site hematoma (5 patients), vesico-ureteral anastomotic stenosis (4 patients) or leak (3 patients), renal artery stenosis (3 patients), lymphocele (3 patients), sepsis (2 patients), renal artery thrombosis (2 patients) and renal vein compression (1 patient). These patients were compared to 177 patients without any surgical complications after KT (Group I). Descriptive data of the demographics of the patients in both groups is shown in Table 2. The donor and recipient age and gender distribution as well as the donor to recipient relationship and HLA matching were comparable between the two groups. The number and type of sensitized patients were also similar between Groups I and II. The differences between the mean fasting blood sugar (91.7 ± 23.1 mg/dl in Group I and 87.1 ± 8.3 mg/dl in Group II), the presence of pre-transplant diabetes (11 patients in group I and 1 patient in Group II) and the mean body mass index (BMI) (25 ± 0.8 in Group I and 23 ± 0.8 in Group II) were statistically not significant between the 2 groups. The in-hospital outcome analysis and the graft function survey revealed that variables which differ significantly between the two groups were: the difference between pre-transplant and post-transplant hemoglobin (Hb) blood level, the post transplant blood transfusions units, the duration of hospital stay, the mean serum creatinine level upon discharge from the hospital and the 6 months graft and patient survival (Table 3). Patients in Group I had lower serum creatinine level upon discharge as well as better 6 months graft and patient survival. However, acute rejection rate and severity as well as the serum creatinine level and the infection rate at 6 months were similar between the 2 groups of patients.
|
|
4. Discussion
- Despite the progressive improvements in KT outcomes related to better immunosuppression[6], surgical complications remain a frequent cause of allograft failure and patient morbidity and mortality in early and late stages following transplantation[5,15]. In a recently published study of 562 re-hospitalized kidney transplant recipients[16], 278 patients (50%) were readmitted during the first 6 months of post-transplant period. The main cause of readmission in the early phase group was surgical complications. Such complications are thought to be costly, to increase morbidity and mortality and to increase the risk of graft loss[17].With an incidence of 2%[18,19] early vascular anastomotic complications[12] can be tremendously morbid to the graft like transplant artery or vein thrombosis leading to early graft loss or spontaneous allograft rupture[20], or even to the patient survival like arterial or venous hemorrhage. The causes are largely technical due to poorly constructed anastomoses, malpositioning of the transplant, rotation of the kidney, or external compression. But other contributory factors have been also reported like recipient hypovolemia and inadequate cardiac output, increased intrarenal pressure as seen with acute tubular necrosis (ATN) or acute rejection, recipient and donor age, recipient and donor vascular pathology, diabetes mellitus, recipients on peritoneal dialysis[21] and, at least in the view of some authors, morbid obesity in repeat transplant recipients[22]. Moreover, numerous hypercoagulable or thrombophilic states identified either inherited or acquired have been also implicated[23-25]. The introduction of CyA-me to clinical practice, usually at doses of 15 mg/kg or more, was associated with an increased incidence of graft thromboses, particularly renal vein thrombosis, in the first week after transplantation[26]. CyA-me subsequently was shown to have procoagulant properties and this is probably a dose response.Occlusion of the renal vein by thrombus at the time of surgery or soon after is an unusual event and invariably associated with a technical problem. More common, at least in past years and with an incidence of 6%, is the seemingly spontaneous event of renal vein thrombosis occurring classically toward the end of the first week of transplantation in an otherwise uncomplicated transplant kidney[27]. Thrombosis prevention strategies include: the minimization of ATN by avoiding prolonged cold and warm ischemia time, careful attention to surgical technique, recipient fluid status, early biopsy diagnosis and aggressive management of vascular and antibody-mediated rejection and the recognition of thrombophilic states by routine screening and directed therapy to reduce the risk of thrombosis and graft loss[28]. Perioperative heparinisation followed by long-term anticoagulation with warfarin has proven efficacy, including successful retransplantation[19,28,29]. Though, the risk of bleeding and hematoma formation seems acceptable in view of the incidence of thrombotic complications. The approach of Oxford transplant unit was to introduce daily aspirin from the time of surgery, the effect of which was to decrease the incidence of renal vein thrombosis from 5.6% to 1.2%[27]. Late vascular complications are represented mainly by transplant renal artery stenosis with an incidence varying widely from 1% to 23% depending on the definition and, more recently, the availability of less invasive diagnostic imaging[30]. It occurs most commonly 3 months to 2 years after transplantation. In a comparatively large series of transplant renal artery stenosis, Voiculescu et.al.[31] reported that most stenoses are identified in the first 6 months. Stenoses at the anastomosis site are more likely to be technical[32] and end-to-side anastomoses may be more of a problem than end-to-end anastomoses[33]. Less morbid complications are represented by hematoma formation which is a common finding after KT in the immediate postoperative period. They can occur spontaneously in an anticoagulated recipient receiving heparin by infusion for prophylaxis against vascular thrombosis[28,29,34] and after percutaneous transplant biopsy.Urological complications occur more frequently ranging between 1% and 15% according to the literature[13, 35-38]. The incidence depends on many factors, in particular duration of follow-up and how broadly urological complications are defined. The majority are confined to ureteric strictures or leaks which are typically caused by either technical errors or ischemia during retrieval. They are more common especially in kidneys with multiple ureters[39].Ureteral leaks are reported in 1% to 3% of renal transplants[35,38]. The two most common causes are ureteral ischemia with necrosis and surgical technical error. Technical errors include: misplacement of ureteral sutures, insufficient ureteral length with tension on the anastomosis, outflow obstruction, unrecognized surgical laceration of the ureter or renal pelvis, acute ureteral obstruction with perforation through a renal calyx and protrusion of a ureteral stent. Leaks resulting from technical errors often occur within the first 24 hours, whereas leaks from necrosis usually occur within the first 14 days. Delayed graft function and older donor age are risk factors for ureteral necrosis[40].In one large series[41] of 1142 patients, urologic complications were present in 8.7% of cases. They were mainly anastomotic leakages occurring mostly in male recipients. Stenosis of the transplant ureter occurs in approximately 3% of transplant recipients[37,42]. The obstruction can be extraluminal (compression from lymphocele or spermatic cord), ureteral (ischemia), or intraluminal (stone, fungal ball, sloughed renal papilla, foreign body). The emerging problem of polyomavirus (BK virus) can produce ureteritis and ultimately ureteral stenosis[43] as well as a case of ureteral obstruction by an Aspegillus infestation, has also been reported[44]. Ureteral stenosis may occur months or years after an otherwise successful transplant. Risks for late ureteral stenosis include advanced donor age, delayed graft function, and kidneys with more than two arteries[45]. Although initial ureteral stenting reduces the incidence of early stenosis, there is no impact on the rate of late ureteral stenosis[46]. The routine use of double-J ureteral stents at the time of kidney transplantation had been controversial. In some series, stents can reduce the incidence of ureteral leaks and early ureteral stenosis[46] and make the early management of leaks easier. Other reports, including prospective randomized trials, have shown no impact[47].Two meta-analyses have addressed the issue of prophylactic routine stenting in renal transplants. Mangus and Haag[48] performed a meta-analysis of 49 published studies, including randomized controlled trials and case studies. These investigators found a significant reduction in ureteric complications with stents in randomized (from 9% to 1.55; P < 0.0001) and case series from 4.8% to 3.2%; p = 0.007) data. In a separate study, Mangus and coworkers[35] found stenting to be cost-effective. Wilson and colleagues[49] analysed data in the Cochrane register of Controlled Trials. They found the relative risk of major urological complications with stents to be 0.24 (95% confidence interval 0.07 to 0.77; p = 0.02). Although urinary tract infections were more common in the stented group, this increase disappeared in patients receiving routine antimicrobial prophylaxis.The incidence of lymphocele in large series is around 2%. But with the advent of ultrasound for routine graft surveillance, together with the realizations that most lymphatic collections remain subclinical and that most resolve spontaneously[50,51], caused the figure to be revised to about 50%.Surgical Site infections (SSI) are also observed in the early post operative period and complicated 4% of cases done in a recent published series, without affecting the overall mortality and morbidity[52]. Mycotic aneurysm caused by candida albicans infestation has been described[53]. Both, SSI and lymphoceles were attributed to the introduction of new immunosuppressant regimens[7,8]. The most recent strong association of mTOR inhibitors with problematic lymphoceles is attributed to their powerful antifibroblastic activity, particularly in obese patients being treated for rejection (BMI > 30 kg/m
 )[54-56].In our study, early surgical post KT complications affected 11.5% of all patients (group II) and were: vascular 47.8 % (5 hematomas, 3 artery stenosis, 2 artery thrombosis and one renal vein compression), urological in 30 % (3 ureteral leak and 4 ureteral stenosis). Other non specific complications have been also observed: 3 patients had lymphoceles and 2 others had sepsis secondary to site infection. Two graft artery thrombosis were described in our study for a rate of 1%. In the first case, the patient was 6 years old having a preemptive transplantation for nephronophtyse. The transplant was done through an intraperitoneal approach where the renal graft was sutured in an end-to-side way with the common right iliac artery of the recipient and the graft vein with the inferior vena cava of the recipient also in end-to-side fashion. In the second case the patient was 51 year old being on peritoneal dialysis because of a polycystic kidney disease. Both thrombosis were diagnosed early during the first week after the transplant and ended by doing a transplant nephrectomy. Coagulation studies were done after the 2 failed procedures and have shown an increase in anti-phopholipids antibodies in the second case only. In the unique case of vein compression, the diagnosis was made following a sudden and unexplained drop in the urine output with surgical site pain on day 2 after the transplant, which was caused by a malpositioning of the kidney graft diagnosed by an urgent surgery and graft recuperation. The routine use of low dose continuous IV heparin infusion started 6 hours after the transplant procedure and continued till the patient discharge from the hospital may explain the low rate of early thrombotic vascular complications in our study. Three cases of graft artery stenosis were diagnosed for a rate of 1.5%. They occur late, on day 120 (1 case) and day 180 (2 cases) after KT. The fact that we performed the arterial anastomosis in an end-to-side fashion using a continuous running 6-0 prolene on one side of the anastomosis and separated stitches on the opposite side will contribute to decrease the tension on the anastomosis lumen reducing the rate of late stenosis. Hematomas were seen in 5 cases (2.5%) and necessitated surgical evacuation in 3 instances. In 4 patients, anticoagulation therapy at high dose was the main reason. No bleeding occurred when mild dose of continuous heparin infusion was given as in our protocol. In the remaining 1 patient, the hematoma was related to technical reason. Urological complications occurred in 7 patients (3.5%). There were 4 ureteral stenosis diagnosed at 46, 62, 69 and 180 days after KT. In 2 cases the patients suffered from severe acute rejection needing anti-Thymocyte globulin - Fresenius (ATG-F) rescue therapy. In the 2 other patients, technical reasons were most probably implicated. The fact that routine ureteral stent is inserted during the procedure, might explain the low rate of ureteral stricture and its late appearance. Moreover, uretero-vesical anastomosis is done using 4-0 Vicryl in separated sutures. Ureteral leaks were present in 3 patients (1.5%) and were related in 1 patient to a bladder outlet obstruction due to a prostate hypertrophy, and in the 2 other patients to a ureteral perforation of its middle segment due to a kidney biopsy on day 23 and to an unexplained perforation on day 14. All 3 patients were reoperated with a primary suture and replacement of a ureteral stent. A transureteral prostatectomy was performed in the first case. In none of the 3 patients, acute rejection or a technical reason was responsible of the ureteral leak. Three cases of lymphocele were described. They have occurred on day 10, 70 and 123. All were treated surgically by peritoneal fenestration and in 1 patient; it was related to Rapamune started early after the transplant because of an acute thrombotic microangiopathy related to Tac. Two patients died from sepsis of unknown origin on day 2 and day 9 after KT. In one patient ATG-F was given intraoperatively as a bolus at a dose of 6 mg/kg, and subsequently severe sepsis occurred without any primary origin. The second patient was urgently transferred to another medical institution for sepsis and died on day 9, 4 days after his hospital discharge. The reason of sepsis was not clear for the medical team.While graft survival is best predicted by creatinine clearance, patients with low Hb blood levels are also considered to be at a high risk for poor graft function, since anemia contributes to mortality and morbidity in kidney transplant patients[57]. In a national survey done in Argentina[58], conducted on 458 patients from different 16 centers, serum creatinine > 2mg/dl and creatinine clearance < 60mL/min were associated with post transplant anemia.In our study, patients with surgical complications after KT needed more blood transfusions and had higher creatinine serum levels upon discharge and at 6 months; mortality was also higher in the complications group (group II). Thus graft survival and patients survival at 6 months, were both correlating with surgical complications. Although becoming rare, surgical complications remain of a great concern, as they affect patient and graft survival and increase morbidity and hospital cost. Despite that we did not identify any specific risk factor; surgical complications may be affected by many parameters related to the surgeon, to the surgical technique and to the recipient. For example, it was reported that night time surgery increases the risk of complications[59]; another study has shown that recipient obesity renders the rate of surgical complications higher[60] and another one postulated that the short time use of ureteral stents decrease urological complications[4].Whether such complications are due to surgical expertise, surgical techniques or recipient factors, surgical complications in kidney transplant recipients should be considered as severe, leading to a decrease in the rate of graft survival and to an increase of the rate of patient mortality and morbidity; and attempts to prevent such complications should be considered.
)[54-56].In our study, early surgical post KT complications affected 11.5% of all patients (group II) and were: vascular 47.8 % (5 hematomas, 3 artery stenosis, 2 artery thrombosis and one renal vein compression), urological in 30 % (3 ureteral leak and 4 ureteral stenosis). Other non specific complications have been also observed: 3 patients had lymphoceles and 2 others had sepsis secondary to site infection. Two graft artery thrombosis were described in our study for a rate of 1%. In the first case, the patient was 6 years old having a preemptive transplantation for nephronophtyse. The transplant was done through an intraperitoneal approach where the renal graft was sutured in an end-to-side way with the common right iliac artery of the recipient and the graft vein with the inferior vena cava of the recipient also in end-to-side fashion. In the second case the patient was 51 year old being on peritoneal dialysis because of a polycystic kidney disease. Both thrombosis were diagnosed early during the first week after the transplant and ended by doing a transplant nephrectomy. Coagulation studies were done after the 2 failed procedures and have shown an increase in anti-phopholipids antibodies in the second case only. In the unique case of vein compression, the diagnosis was made following a sudden and unexplained drop in the urine output with surgical site pain on day 2 after the transplant, which was caused by a malpositioning of the kidney graft diagnosed by an urgent surgery and graft recuperation. The routine use of low dose continuous IV heparin infusion started 6 hours after the transplant procedure and continued till the patient discharge from the hospital may explain the low rate of early thrombotic vascular complications in our study. Three cases of graft artery stenosis were diagnosed for a rate of 1.5%. They occur late, on day 120 (1 case) and day 180 (2 cases) after KT. The fact that we performed the arterial anastomosis in an end-to-side fashion using a continuous running 6-0 prolene on one side of the anastomosis and separated stitches on the opposite side will contribute to decrease the tension on the anastomosis lumen reducing the rate of late stenosis. Hematomas were seen in 5 cases (2.5%) and necessitated surgical evacuation in 3 instances. In 4 patients, anticoagulation therapy at high dose was the main reason. No bleeding occurred when mild dose of continuous heparin infusion was given as in our protocol. In the remaining 1 patient, the hematoma was related to technical reason. Urological complications occurred in 7 patients (3.5%). There were 4 ureteral stenosis diagnosed at 46, 62, 69 and 180 days after KT. In 2 cases the patients suffered from severe acute rejection needing anti-Thymocyte globulin - Fresenius (ATG-F) rescue therapy. In the 2 other patients, technical reasons were most probably implicated. The fact that routine ureteral stent is inserted during the procedure, might explain the low rate of ureteral stricture and its late appearance. Moreover, uretero-vesical anastomosis is done using 4-0 Vicryl in separated sutures. Ureteral leaks were present in 3 patients (1.5%) and were related in 1 patient to a bladder outlet obstruction due to a prostate hypertrophy, and in the 2 other patients to a ureteral perforation of its middle segment due to a kidney biopsy on day 23 and to an unexplained perforation on day 14. All 3 patients were reoperated with a primary suture and replacement of a ureteral stent. A transureteral prostatectomy was performed in the first case. In none of the 3 patients, acute rejection or a technical reason was responsible of the ureteral leak. Three cases of lymphocele were described. They have occurred on day 10, 70 and 123. All were treated surgically by peritoneal fenestration and in 1 patient; it was related to Rapamune started early after the transplant because of an acute thrombotic microangiopathy related to Tac. Two patients died from sepsis of unknown origin on day 2 and day 9 after KT. In one patient ATG-F was given intraoperatively as a bolus at a dose of 6 mg/kg, and subsequently severe sepsis occurred without any primary origin. The second patient was urgently transferred to another medical institution for sepsis and died on day 9, 4 days after his hospital discharge. The reason of sepsis was not clear for the medical team.While graft survival is best predicted by creatinine clearance, patients with low Hb blood levels are also considered to be at a high risk for poor graft function, since anemia contributes to mortality and morbidity in kidney transplant patients[57]. In a national survey done in Argentina[58], conducted on 458 patients from different 16 centers, serum creatinine > 2mg/dl and creatinine clearance < 60mL/min were associated with post transplant anemia.In our study, patients with surgical complications after KT needed more blood transfusions and had higher creatinine serum levels upon discharge and at 6 months; mortality was also higher in the complications group (group II). Thus graft survival and patients survival at 6 months, were both correlating with surgical complications. Although becoming rare, surgical complications remain of a great concern, as they affect patient and graft survival and increase morbidity and hospital cost. Despite that we did not identify any specific risk factor; surgical complications may be affected by many parameters related to the surgeon, to the surgical technique and to the recipient. For example, it was reported that night time surgery increases the risk of complications[59]; another study has shown that recipient obesity renders the rate of surgical complications higher[60] and another one postulated that the short time use of ureteral stents decrease urological complications[4].Whether such complications are due to surgical expertise, surgical techniques or recipient factors, surgical complications in kidney transplant recipients should be considered as severe, leading to a decrease in the rate of graft survival and to an increase of the rate of patient mortality and morbidity; and attempts to prevent such complications should be considered. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML