-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
2012; 2(2): 1-3
doi: 10.5923/j.ajmms.20120202.01
Total Antioxidant Capacity in Serum of Plasmodium falciparum Malarial Infected Patients Receiving Artemisinin-Based Combination Therapy
Onyesom I. 1, Osioma E. 2, Omoghene O. 1
1Department of Medical Biochemistry, Delta State University, Abraka, Nigeria
2Department of Biochemistry, Faculty of Science, University of Ilorin, Nigeria
Correspondence to: Onyesom I. , Department of Medical Biochemistry, Delta State University, Abraka, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
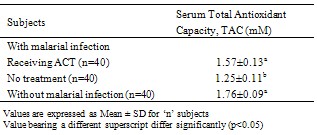
This study was undertaken to assess the effect of artemisin-based combination therapy (ACT) on serum total antioxidant capacity (TAC). Eighty (80) consenting patients infected with P. falciparum malaria (40 receiving ACT and 40 yet to be treated) and 40 uninfected individuals in apparent good health were selected. Serum total antioxidant capacity was determined by the trolox equivalent method as earlier described. Results show that P. falciparum malarial infection significantly reduced (p < 0.05) serum TAC (1.25±0.11mM) when compared with values obtained from the uninfected, healthy subjects (1.76±0.09mM) and the infected patients receiving ACT (1.57±0.13Mm). ACT treatment enhanced serum TAC by 25.6% in comparison with the value obtained from malarial infected patients yet to receive treatment. Thus, treatment of P. falciparum malarial infection with ACT significantly (p<0.05) improved the capacity of antioxidants in serum of infected patients, and this represents an enhanced defense against oxidants including reactive oxygen species, ROS, generated by the malarial parasite’s –induced stimulation of the immune response.
Keywords: Plasmodium falciparum, Malaria, Total antioxidant capacity, Artemisinin, Patients
1. Introduction
- Malaria is a mosquito-borne infectious disease of the blood caused by the parasite, Plasmodium sp. It spreads through the bite of infected female Anopheles mosquito and is endemic in tropical and sub-tropical regions including parts of America, Asia and Africa. Malaria represents a medical emergence because it may rapidly progress to complication and death without prompt and appropriate treatment[1]. The malarial infection remains a devastating global problem with an estimated 300 – 500 million cases occurring annually[2], killing between 1-3 million people each year.Malarial infection has the ability to activate the immune system which causes the release of reactive oxygen species (ROS) with the potency of inducing oxidative damage and cell destruction[3]. The body however, has a number of mechanisms to minimize the cellular effects of ROS. These defense mechanisms include the production of antioxidants.Antioxidants may be obtained from diets or synthesized in the body through various intracellular mechanisms. An antioxidant could be preventive or chain breaking. Preventive antioxidants (catalase, glutathione peroxidase, EDTA, vitamin C) inhibit the initial production of free radicals including ROS, while chain breaking antioxidants (superoxide dismutase, uric acid, vitamin E) inhibit the damaging phase of ROS[4].Recent biochemical advances have been focused on antioxidants and their potency in minimizing the damaging effects of free radicals, as well as their roles in potentiating drug efficacy. Some biochemical changes regarding the oxidant and antioxidant levels in malaria infected patients have been observed[5]. Negative correlation between Plasmodium falciparum load and serum antioxidant vitamins (A, C, and E) has been demonstrated among malarial infected childrens[6]. Antagonistic effect of vitamin E on the efficacy of artesunate against Plasmodium berghei infection has been reported in mice[7]. WHO[8] discouraged the administration of single drugs alone (monotherapy) for the treatment of malaria due to parasites’ development of resistance to drugs in recent years. According to WHO[8], therapies that combine artemisinin (a sesquiterpene lactone containing an unusual peroxide bridge from the plant, Artemisa annua) and its derivatives with some other non- artemisinin-based antimalarial drugs are the preferred treatment for malaria, and are effective and well tolerated by patients. This ofcourse, gave birth to the concept of artemisinin-based combination therapy (ACTs) which has now become a standard treatment for the management of malaria caused by all the species of Plasmodium[9].For several reasons (increased effectiveness, low resis- tance, few side effects, high tolerance)[10], the use of different brands of ACTs[11] has become very popular, yet reports on antioxidant capacity of malarial patients receiving ACT treatment have remained scarce in Nigeria. This paper therefore reports the total antioxidant capacity in serum of P. falciparum malaria infected patients receiving different brands of ACT in Abraka, a university community in Delta State, Nigeria.
2. Materials and Methods
- Subjects: One hundred and twenty (120) consenting individuals, (males/females) between the ages of 15-44 years were selected and divided into three groups (n=40/group). The first group had P. falciparum malarial infection and were almost completing ACT treatment. The second group also had P. falciparum malarial infection but were yet to undergo treatment, while the third group were apparently healthy individuals with no malarial infection. The patients were obtained from some clinics and hospitals in Abraka. The presence or absence of P. falciparum infection was confirmed by the Giemsa stain technique using both thin and thick blood smears.Sample collection and handling: Fasting blood sample (2ml) was collected from each subject using sterile hypodemic needle and syringe into plain sterile bottle. The collected whole blood sample was allowed to clot and then centrifuged at 1200xg for 5 min at room temperature (29-32℃).The resulting supernatant (serum) was decanted into a bijou bottle and stored frozen until required for analysis which was done within 48 hours.Total antioxidant Capacity (TAC) Assay: Serum TAC was determined using the trolox equivalent assay method [12]. The reagents were supplied in commercial kit by Cayman Chemicals, USA.Statistics: The data are presented as Mean ±SD. Statistical significance between groups was assessed by one -way analysis of variance (ANOVA) followed by Duncan’s test for multiple comparisons and statistical significant difference was established at p <0.05. The SPSS package version 16 was used for the analysis as described[13].
3. Results
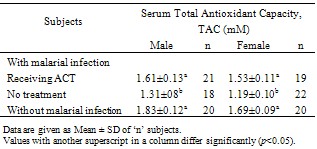
- The results obtained from this investigation into the total antioxidant capacity (TAC) in serum of P. falciparum malarial infected patients receiving artemisinin-based combination therapy (ACT) are given in Tables 1-3.Experimental evidence (Table 1) indicates that malarial infection significantly (p<0.05) reduces serum total antioxidant capacity, but ACT treatment of the infection ameliorates the condition by elevating serum TAC level by 25.6%.The serum total antioxidant capacity for the healthy male subjects without malarial infection was higher (p>0.05) when compared with the female counterpart, but malarial infection significantly (p<0.05) reduced the serum TAC in both gender, though effect was more marked among the female patients. Treatment of the malarial infection with ACT was seen to raise TAC by 22.9% and 28.6% among the male and female patients, respectively, from comparable values for those infected with malaria but yet to commence treatment.
|
|
|
4. Discussion
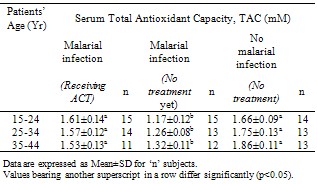
- In this study, the total antioxidant capacity in serum of P. falciparum malarial infected patients receiving ACT was determined and the estimated values were compared with the figures obtained from P. falciparum malarial infected patients (yet to be treated) and uninfected, healthy individuals. Evidence shows that P. falciparum malarial infection significantly (p<0.05) reduced serum TAC, but ACT treatment significantly (p <0.05) improved the serum antioxidant capacity (Table 1) especially among the female patients (Table 2) and individuals between 15 – 24 years (Table 3).Infection with P. falciparum has been observed to stimulate the immune system and this has been established to increase the production of free radicals, especially reactive oxygen species, ROS[3]. Free radicals in excess are known to induce lipid peroxidation and cell damage[14]. However, the body has a number of defence mechanisms which include the production and utilization of antioxidants[4]. Therefore, free radical challenge could induce reduction in antioxidant levels as observed in this study. This further strengthens the involvement of antioxidants in the defense of ROS induced damages. Our observation is consistent with earlier studies[5]. This group of workers reported an imbalance in the oxidant and antioxidant levels in serum of malarial infected patients.Nevertheless, treatment of P. falciparum malarial infection with ACT boosted the capacity of serum antioxidants. The mechanism(s) of this ACT – enhanced antioxidant capacity in serum is complex, but it seems that ACT may elicit the production of antioxidants. Treatment of malarial infection with chloroquin, an anti-malarial drug, significantly increased (p<0.05) the amounts and activities of SOD and catalase in experimental animals[15]. Catalase and SOD are two respective important preventive and chain breaking antioxidants[5]. Since ACT drugs target and destroy malarial parasite infected erythrocytes, the immune system produces antioxidants to metabolize the existence of these drugs, thereby causing an increased production and concentration of total antioxidants.Treatment of P. falciparum malarial infection with ACT seems to enhance antioxidants’ defense against the parasites’ induced increase in ROS reactivities and possible associated damages. This defense was more marked among the female patients and individuals between 15-24 years.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML