-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2012; 2(1): 36-39
doi:10.5923/j.ajmms.20120201.08
A Simple and Effective Methodology for the Sulfonylation of Alcohols and Aniline under Solvent Free Condition at Room Temperature
Reza Tayebee, Farzaneh Nehzat
Department of Chemistry, School of Sciences, Sabzevar Tarbiat Moallem University, Sabzevar, 96179-76487, Iran
Correspondence to: Reza Tayebee, Department of Chemistry, School of Sciences, Sabzevar Tarbiat Moallem University, Sabzevar, 96179-76487, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Sulfonylation is an important reaction in the synthesis of naturally occurring bioactive molecules and is one of the most important methods for the protection of alcohols and amines. However, many efforts have been made towards the development of novel methods for the preparation of the target compounds. Most of these methods are involved the reaction of amine and alcohol compounds with sulfonyl chlorides by using an organic solvent, a base, and under high temperatures. Herein, we introduce our findings on the sulfonylation of aniline, 4-nitroaniline, and some alcohols bearing electron donating or withdrawing substituents with p-toluenesulfonyl chloride under very simple conditions in the absence of any expensive additive, as catalyst, and under solvent free condition at room temperature.
Keywords: Sulfonylation , Aniline, Alcohol, Solvent Free, P-Toluenesulfonyl Chloride
Cite this paper: Reza Tayebee, Farzaneh Nehzat, A Simple and Effective Methodology for the Sulfonylation of Alcohols and Aniline under Solvent Free Condition at Room Temperature, American Journal of Medicine and Medical Sciences, Vol. 2 No. 1, 2012, pp. 36-39. doi: 10.5923/j.ajmms.20120201.08.
1. Introduction
- The development of new, simple, environmentally-benign, and economically attractive chemical processes or methodologies for widely used organic compounds is in great demand. Organosulfones are one of the most familiar compounds in organic synthesis and industry which have several pharmaceutical applications[1,2]. One of these useful compounds are 1, 2-diarylsulfones, as effective drugs against leishmaniasis, malaria, and infections in patients with AIDS discoid lupus erythematosus[3,4]. Sulfonamide derivatives constitute the most important classes of pharmaceuticals. Antibacterial agents with a sulfonamide structure, such as sulfadiazine, and hydrochlorothiazide, have been therapeutically used for many decades[5-7]. For example, many drugs with a sulfonamide structure are antihypertensive bosentan, have the antiviral HIV protease inhibitor amprenavir, and the phophodiesterase-5 inhibitor sildenafil[15-18]. In addition, numerous sulfonamide derivatives have been used in preclinical development. The sulfonamide partial structure appears to belong to the so-called ‘‘privileged structures’’ in medicinal chemistry, and showed several pharmacokinetic properties including metabolic stability. Moreover, 3,4-diaryl and aryl/alkyl sulfones could be synthesized by conventional Friedel-Crafts type sulfonylation of aromatic compounds by sulfonyl halides in the presence of Lewis acids such as AlCl3, BF3, triflic acid/BiCl3, Zn-exchanged zeolites, Fe(III)-exchanged montmorillonite clay, scandium and lanthanide(III) salts[8], and Cu(OTf)2 or Sn(OTf)2[9]. The other branch of organosulfones are sulfonamides which were used widely in pharmaceutical compounds because of their wide range of biological activities such as anticancer, anti-inflammatory and antiviral functions. Moreover, another important application of sulfonamides is their function as protecting groups of OH or NH functionalities for easy removal under mild conditions [10-13]. Therefore, there are significant demands by the pharmaceutical industry for cheap, efficient and environmentally friendly procedures for the synthesis of these valuable compounds. Even though, many synthetic methods have been reported, the sulfonylation of amines with sulfonyl chlorides in the presence of a base is still being used as the method of choice because of high efficiency and simplicity of the reaction. However, this approach is limited by the formation of undesired di-sulfonamides with primary amines and by the need of harsh reaction conditions for less nucleophilic amines such as anilines[14]. Additionally, side reactions take place in the presence of a base. In continuation of our studies on solvent-free organic reactions[19], we report herein an efficient method for the synthesis of sulfonamides via the condensation of amines and alcohols with p-toluenesulfonyl chloride in the presence or absence of catalyst (zinc oxide) under solvent-free conditions at room temperature (Scheme 1). In this communication, we find that the sulfonylation reactions of alcohols, aniline, and 4-nitroaniline toke place under solvent free condition.
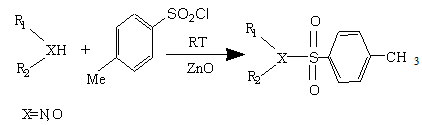 | Scheme 1. |
2. Experimental
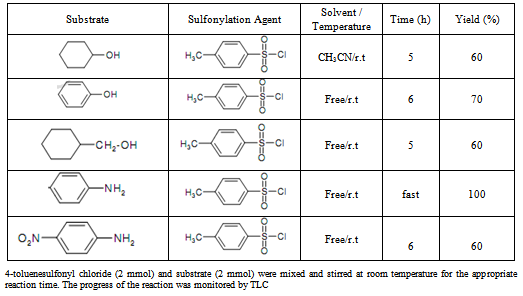
- Chemicals such as anilines, p-toluene sulfonyl chloride, alcohols, metal oxides, and other chemicals were purchased from Fluka, Merck and Aldrich Chemical Companies. The sulfonylation products were characterized by comparison of their spectral (IR, 1H-NMR), TLC and physical data with authentic samples[20]. Infrared spectra were run on a Shimadzu Model 8700 Fourier transform spectrophotometer. The heteropolyacid catalysts were prepared and characterized according to the literature procedures.Typical procedure for the sulfonylation of aniline4-toluenesulfonyl chloride (2 mmol) and aniline (2 mmol) were mixed and stirred at room temperature for the appropriate reaction time (Table 1). The progress of the reaction was monitored by TLC. Spectral data of some selected compounds[21-25]o-Phenyl-p-toluenesulfonamide: White crystal, IR (neat, cm-1): 3375, 1585, 1284, 1144 cm-1; 1H NMR (CDCl3, 250 MHz) δ 2.38 (s, 3H), 6.30 (s, 1H), 6.90 (d, 2H), 7.37 (d, 2H), 7.79 (d, 4H); MS (m/e) 249 (M+, 100, base peak), 141 (45.8), 108 (43.4), 91 (32.6), 65 (66.2), 43 (54.7)N-Phenyl-p-toluenesulfonamide: IR (KBr, cm-1): 3208 (NH), 1361, 1149 (SO2), 1H NMR (300 MHz, CDCl3): d 7.18– 7.39 (10H, Ar), 9.52 (s, 1H, NH, D2O exchangeable), MS: m/z = 233 (M+). N-(4-nitroPhenyl)-p-toluenesulfonamide: IR ( KBr, cm-1): 539, 679, 811, 910, 1090, 1159, 1509, 1597, 1611, 3268 cm-1, 1H NMR (300MHz, CDCl3): 1.7 (s, 1H, NH); 2.4 (s, 3H, CH3); 3.8 (s, 3H, OCH3); 6.8(d, 2H, Ar), 7.0(d, 2H, Ar)., 7.2(d, 2H, Ar), 7.6(d, 2H, Ar)
3. Results and Discussion
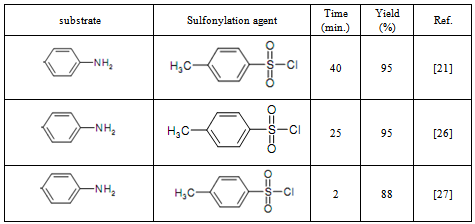
- In order to find the optimized condition for the reaction, condensation of p-toluenesulfonyl chloride with aniline was studied as a model reaction under neat condition at the temperature range from 20 to 100℃. It was found that the temperature change did not affect the reaction yield and the desired product was formed immediately at room temperature. Sulfonylation of anilin (2 mmole) with p- toluenesulfonyl chloride (2 mmol)/benzene sulfonyl chloride (2mmol) at room temperature was studied. It was found that, this reaction is strongly exothermic and can be achieved without using of catalyst under solvent free condition and gave a moderate yield of the corresponding sulfonamide, whereas, the use of small amount of catalyst is necessary for amines with electron withdrawing groups. To find out the catalyst effect on the reaction, we used a little amount of ZnO (1 mol%) in the reaction of some alcohols with p- toluenesulfonyl chloride. The results are summarized in Table 1. It should be noted that elongation of the reaction time did not affect the yield of the product.In order to show the generality of the method, the reaction of structurally different alcohols, aniline, and 4-nitroaniline with p-toluenesulfonyl chloride was examined. The results indicated all reactions proceeded effectively and the desired organosulfones were obtained in good to excellent yields. It was observed that the electronic factors played a significant role in these reactions. Aromatic amines reacted faster than alcohols and provided the corresponding sulfonamides in higher yields. The chemoselectivity of the method was also noteworthy. Whilst, sulfonylation of molecule having both NH2 and OH groups afforded the corresponding sulfonamide in high yield and the OH group remained intact.
|
|
4. Conclusions
- We have developed an economical and green process for the catalytic synthesis of some organosulfones in the presence of zinc oxide under solvent free conditions. Findings showed that sulfonylation of aniline would be achieved in absence of catalyst at room temperature under solvent free condition.
References
| [1] | N. S. Simpkins, 1993, Sulfones in organic synthesis; Pergamon Press: Oxford |
| [2] | M. Roy, 1985, Ullmann’s encyclopedia of industrial chemistry; Gerhartz, W., Ed.; VCH: Weinheim, V.A 25, pp 487-501 |
| [3] | A. Scozzafava, T. Owa, A. Mastrolorenzo, C.T. Supuran, 2003, Anticancer and antiviral sulfonamides. Curr. Med. Chem. 10, 925 |
| [4] | G. Wozel, 1989, The story of sulfones in tropical medicine and dermatology. Int. J. Dermatol., 28, 17 |
| [5] | I.C. Richards, P.S. Thomas, 1990, Sulfonylation of aromatic compounds with sulfonic acids using silica gel-supported AlCl3 as a heterogeneous Lewis acid catalyst. Pestic. Sci., 30, 275 |
| [6] | C.J. Dinsmore, T.M. Williams, T.J. O’Neill, D. Liu, E. Rands, J.C. Culberson, R.B. Lobell, K.S. Koblan, N.E. Kohl, J.B. Gibbs, A.I. Oliff, S.L. Graham, C.D. Hartman, 1999, Imidazole-containing diarylether and diarylsulfone inhibitors of farnesyl-protein transferase. Bioorg. Med. Chem. Lett., 9, 3301 |
| [7] | D.J. Abraham (Ed.), 2003, Burger’s medicinal chemistry & drug discovery, John Wiley and Sons, Hoboken, NJ |
| [8] | C.G. Frost, J.P. Hartley, D. Griffin, 2002, Counterion effects in indium-catalysed aromatic electrophilic substitution reactions. Tetrahedron Lett., 43, 4789 |
| [9] | R.P. Singh, R.M. Kamble, K.L. Chandra, P. Saravanane, V.K. Singh , 2001, An efficient method for aromatic Friedel–Crafts alkylation, acylation, benzoylation, and sulfonylation reactions. Tetrahedron, 57, 241 |
| [10] | H. Nishida, T. Hamada, Yonemitsu, 1988, hydrolysis of tosyl esters initiated by an electron transfer from photoexcited electron-rich aromatic compounds. J. Org. Chem., 53, 3386 |
| [11] | W. Yuan, K. Fearson, M.H. Gelb, 1989, Synthesis of sulfur-substituted phospholipid analogs as mechanistic probes of phospholipase A2 catalysis. J. Org. Chem, 54, 906 |
| [12] | J.F. O’Connell, H. Rapoport, 1992, 1-Benzenesulfonyl- and 1-p-toluenesulfonyl-3-methylimidazolium triflates: efficient reagents for the preparation of arylsulfonamides and arylsulfonates. J. Org. Chem., 57, 4775 |
| [13] | S. Chandrasekhar, S. Mohapatra, 1998, Asymmetric synthesis of anti-convulsive drug (S)-Vigabatrin. Tetrahedron Lett., 39, 695 |
| [14] | A. Yasuhara, M. Kameda, Sakamoto, 1999, Selective monodesulfonylation of N,N-disulfonylarylamines with tetrabutylammonium fluoride. Chem. Pharm. Bull. 47, 809 |
| [15] | C.T. Supuran, A. Casini, A. Scozzafava, 2003, Protease inhibitors of the sulfonamide type: Anticancer, antiinflammatory, and antiviral agents. Med. Res. Rev. 5, 535 |
| [16] | A. Scozzafava, T. Owa, A. Mastrolorenzo, C.T. Supuran, 2003, Anticancer and antiviral Sulfonamides. Curr. Med. Chem. 10, 925. |
| [17] | J.B. McMohan, R.J. Gulakowsky, O.S. Weislow, R.J. Schultz, V.L. Narayanan, D.J. Clanton, R. Pedemonte, F.W. Wassmundt, R.W. Buckheit Jr., W.D. Decker, E.L. White, J.P. Bader, M.R. Boyd, 1993, Diarylsulfones, a new chemical class of nonnucleoside antiviral inhibitors of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents. Chemother. 37 ,754 |
| [18] | K. Ohta, S. Itoh, J. Yamada, K. Masumoto, H. Yoshikawa, Y. Ishida, 1993, An eco-friendly N-sulfonylation of amines using stable and reusable Zn–Al–hydrotalcite solid base catalyst under ultrasound irradiation. J. Pest. Sci. 18, 183 |
| [19] | R. Tayebee, F. Cheravi, M. Mirzaee, M.M. Amini, 2010, Commercial zinc oxide (Zn2+) as an efficient and environmentally benign catalyst for homogeneous benzoylation of hydroxyl functional groups. Chin. J. Chem, 28, 1247 |
| [20] | R.P. Singh, R.M. Kamble, K.L. Chandra, P. Saravanane, V. K. Singh, 2001, . Tetrahedron, 57, 241 |
| [21] | M. Jafarpour, A. Rezaeifard, M. Aliabadi, 2009, Catalytic activity of silica gel in the synthesis of sulfonamides under mild and solvent-free conditions, Appl. Catal. A: Gen. 358 49 |
| [22] | A.R. Massah, D. Azadi, H. Aliyan, A.R. Momeni, H. Javaherian Naghash, F. Kazemi, 2008, An efficient method for the synthesis of N-acylsulfonamides: One-pot sulfonylation and acylation of primary arylamines under solvent-free conditions, Monatsh. Chem. 139, 233 |
| [23] | H. Sharghi, Z. Shahsavari-Fard, 2005, Al2O3/MeSO3H (AMA) a useful system for direct sulfonylation of phenols with p-toluenesulfonic acid, J. Iran. Chem. Soc., 2, 47 |
| [24] | R.P. Singh, R.M. Kamble, K.L. Chandra, P. Saravanan, V. K, Singh, 2001, An efficient method for aromatic Friedel–Crafts alkylation, acylation, benzoylation, and sulfonylation reactions, Tetrahedron 57, 241 |
| [25] | M.V. Alexander, A.C. Khandekar, S.D. Samant, 2004, Sulfonylation reactions of aromatics using FeCl3-based ionic liquids, J. Mol. Cat. A: Chem. 223, 75 |
| [26] | J. A. Kamal, E.S. Reddy, D.V. Bharathi, 2008, Base-free monosulfonylation of amines using tosyl or mesyl chloride in water, Tetrahdron Lett. 49, 348 |
| [27] | G.A. Meshram, V.D. Patil, 2009, A simple and efficient method for sulfonylation of amines, alcohols and phenols with cupric oxide under mild conditions. 50, 2009, 1117 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML