-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2012; 2(1): 5-11
doi:10.5923/j.ajmms.20120201.02
Effect of Selenium on Carbimazole-Induced Histopathological and Histochemical Alterations in Prostate of Albino Rats
Saber A. Sakr, Hoda A. Mahran, Amany E. Nofal
Zoology Department, Faculty of Science, Menoufia University, Shebin El-kom, Egypt
Correspondence to: Saber A. Sakr, Zoology Department, Faculty of Science, Menoufia University, Shebin El-kom, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Carbimazole is an antithyroid drug used in treatment of hyperthyroidism. The present study is undertaken to evaluate the effect of carbimazole in prostate of albino rats and the ameliorative role of selenium. Treating rats with carbimazole(1.35mg/Kg b.w) daily for 8 weeks caused distinct histological alterations in prostate gland compared with control group. The epithelial cells of prostatic acini exhibited degeneration. Hyperplasia were projected into the lumens of the prostatic acini or to the outside stroma. Most of the intertubular blood vessels were congested. Histomorphological results showed that the heights of the epithelial cells were significantly reduced and the thickness of smooth muscle layer increased. Prostate gland of animals treated with carbimazole showed gradual decrease in the polysaccharide, protein and nucleic acids contents. These results were time-dependent. Treating animals with carbimazole and selenium showed an improvement in the histological structure as well as histochemical components of the prostate gland. It is suggested that the ameliorative effect of selenium against the histological and histochemical changes induced by carbimazole may be due to its antioxidant properties.
Keywords: Carbimazole, Selenium, Prostate, Histology, Histochemistry, Rat
Cite this paper: Saber A. Sakr, Hoda A. Mahran, Amany E. Nofal, Effect of Selenium on Carbimazole-Induced Histopathological and Histochemical Alterations in Prostate of Albino Rats, American Journal of Medicine and Medical Sciences, Vol. 2 No. 1, 2012, pp. 5-11. doi: 10.5923/j.ajmms.20120201.02.
Article Outline
1. Introduction
- Carbimazole is an antithyroid drug widely prescribed for treatment of hyperthyroidism. It is a 3-carbethoxy methimazole derivative, metabolized to methimazole in the liver. Serum thyroxine, thyroid-stimulating hormone and thyrotropin-bindinginhibitory immunoglobulins are decreased after 2, 4 and 6 weeks of carbimazole treatment[1]. Frenais et al.[2] reported that carbimazole is a common oral treatment for hyperthyroidism in cats. On the other hand, the use of carbimazole was associated with various adverse effects. Ali et al.[3] showed that carbimazole produced mild necrosis of renal tubules in rats. Marazuela et al.[4] mentioned that carbimazole was capable of inducing acute pancreatitis and cholestatic hepatitis in 33-year old female. Zaidi et al.[5] reported that carbimazole administration even in therapeutic dose during pregnancy and lactation resulted into alteration of the thyroid microstructure of the newborn. Pulmonary haemorrhage and necrotizing glomerulonephritis were associated with carbimazole therapy[6]. Vilchez et al.[7] reported that carbimazole therapy caused both minor (e.g. pruritus, rash, urticaria, fever and arthralgias) and potentially life-threatening (e.g, agranulocytosis, hepatotoxi-city with severe cholestatic jaundice) effects.Oxidants and antioxidants have attracted widespread interest in nutrition research, biology and medicine. Selenium is an essential element important in many biochemical and physiological processes including the biosynthesis of coenzyme Q (a component of mitochondrial electron transport systems), regulation of ion fluxes across membranes, maintenance of the integrity of keratins, stimulation of antibody synthesis, and activation of glutathione peroxidase[8]. [9] reported that the plasma selenium levels indicate the amount of circulating selenoproteins and selenoenzymes which are important for modulating the immune system and also for thyroid hormone metabolism. Sodium selenite is commonly used as a direct supplement for the treatment of selenium deficiency. Selenium had antiperoxidative effect and capacity to prevent cancer[10]. Recent studies have noted an inverse relationship between selenium status and cancer risk in several tissues, including the esophagus, stomach, lung, and prostate[11]. et al.[12] did not support the hypotheses that selenium is potent cancer chemopreventive agent in the prostate of rat. et al.[13] reported that selenium played a beneficial role for prevention of cisplatin hepatotoxicity in mice. Selenium has a protective effect against rat liver and kidney damage induced by mercury chloride[14] and by cadmium[15]. Sakr et al.[16] found that selenium ameliorated the testicular damage and oxidative stress induced by carbimazole in albino rats. The present work aims to investigate the effect of selenium on the histological and histochemical alterations induced by carbimazole in prostate of albino rats.
2. Material and Methods
2.1. Animals
- Sexually mature male albino rats(Rattus norvegicus) weighing 125 ± 5 g and aged 15 weeks were purchased from the breeding center of experimental animals at Helwan University, Helwan, Egypt. The animals were kept in the laboratory under constant temperature (25±1°C) for at least one week before and along the period of the experimental work. They were maintained on a standard rodent diet composed of 55% corn starch, 20% casein, 15% corn oil, 5% salt mixture and 5% vitaminized starch (Egyptian Company of Oils and Soap Kafr-Elzayat, Egypt). Water was available ad libitum.
2.2. Experimental Design
- The animals were divided into 4 groups (20 animals each). Group1: animals of this group were served as normal control. Group 2: animals of this group were orally given carbimazole (1.35 mg/Kg b.w, equivalent to the therapeutic dose for human[17] ) dissolved in water, daily for 8 weeks. Group 3: animals of this group were orally given sodium selenite (10 μg/Kg b.w) dissolved in water, daily for 8 weeks. Group 4: animals of this group were orally administered carbimazole (1.35mg/Kg b.w) and sodium selenite (10μg/Kg b.w)[18] for 8 weeks. All the experiments were done in compliance with the Guide for the Care and Use of Laboratory animals. The treated animals were sacrificed by cervical decapitation after 4 and 8 weeks of treatment.
2.3. Histological and Histochemical Examination
- Ten rats were sacrificed from treated and control groups after 4 and 8 weeks. Their prostate glands were excised. For histological study, prostate glands were fixed in alcoholic Bouin’s fluid, dehydrated in ethyl alcohol, cleared in xylol and embedded in paraffin wax. Sections of five micrometers thickness were cut and stained with haematoxylin and eosin. For histochemical study, specimens were fixed in Carnoy’s fluid. Periodic acid Schiff’s reaction[19] was used for demonstration of polysaccharides. Total proteins were detected using the mercury bromophenol blue method[20]. Nucleic acids (DNA&RNA) were determined using Schiff-methylene blue method[21]. The epithelial height and thickness of smooth muscle layer were measured in sections of the acinus stained with H and E. All data were obtained from 10 random microscopic fields per animal at X 100 objective.
2.4. Statistical Analysis
- The results were expressed as mean ± SD of different groups. The differences between the mean values were evaluated by ANOVA followed by Student’s “t” test using Minitab 12 computer program (Minitab Inc., State Collage, PA).
3. Results
3.1. Morphometrical Results
- Figures (1a&b) showed the morphometric changes in the epithelial heights and thickness of smooth muscle layer in the prostate acini. Treating animals with carbimazole for 8 weeks showed significant decrease (P<0.05) in the cell epithelial heights. On the other hand, animals treated with carbimazole and selenium for the same period showed significant increase in the epithelial heights in comparison with carbimazole group. The thickness of the smooth muscle layer increased significantly in rats treated with carbimazole. No significant changes were recorded in heights of epithelial cells or thickness of smooth muscle layer in selenium-treated rats compared with control animals.
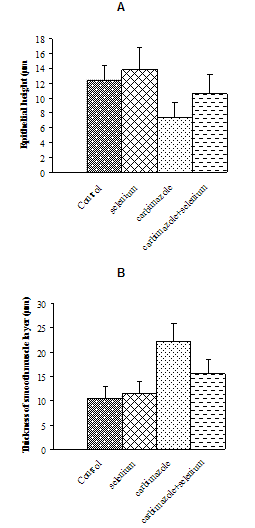 | Figure 1. Change in cells epithelial heights (A) and thickness of smooth muscle layers (B) in different animal groups |
3.2. Histopathological Results
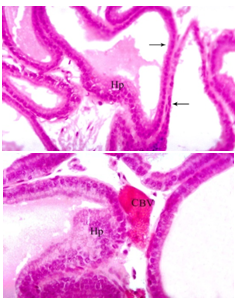
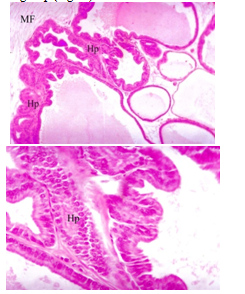 | Figure 4. Sections in prostate gland of a rat daily treated with carbimazole for 8 weeks showing hyperplasia (Hp) with thick muscle fibers (MF), (H&E.,(a) X 100 and (b) X 400 ) |
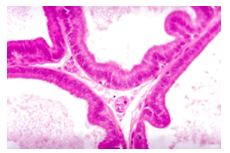 | Figure 5. Section of prostatic acini of a rat daily treated with carbimazole and selenium for 8 weeks showing normal acini with normal folded epithelial layers, (H&E., X 400) |
3.3. Histochemical results
3.3.1. Polysaccharides
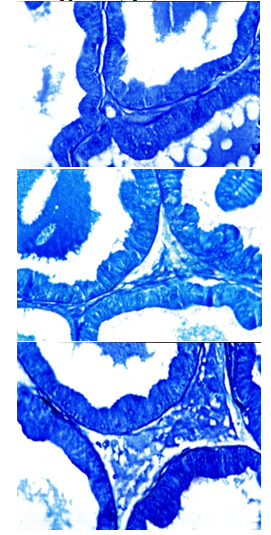
- Prostate of control rats revealed that the cytoplasm of epithelial layers had a moderate PAS-positive materials while their nuclei were negatively stained. The brush borders of the epithelial layers, prostatic concretion and stroma appeared with strong PAS reaction (Fig. 6a). Prostate of carbimazole-treated animals revealed gradual decrease of PAS-positive materials and reached its maximum after 8 weeks (Fig. 6b). Examination of Prostate glands of rats treated with carbimazole followed by selenium showed an increase of the polysaccharide content (Fig. 6c).
3.3.2. Total Proteins
- Total proteins appeared in the prostate of control rats as deeply stained granules in the cytoplasm and nuclei of the epithelial cells (Fig. 7 a). Animals treated with carbimazole showed a noticeable decrease in the protein content (Fig. 7b). Animals treated with carbimazole and selenium revealed that the epithelial cells contained a somewhat normal content of total proteins (Fig. 7c).
3.3.3. Nucleic Acids (DNA & RNA)
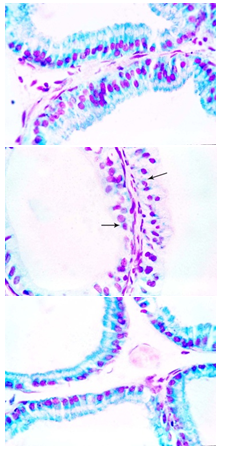
- In the epithelial cells of prostate of control rat, DNA present in the nuclei as granules of magenta colour, while RNA was shown as blue fine granules in the cytoplasm of these cells (Fig. 8a). RNA and DNA-containing particles were markedly decreased in the epithelial cells after 8 weeks of carbimazole treatment (Fig. 8b). Animals treated with carbimazole and selenium showed an increase in both RNA and DNA-containing particles (Fig.8c).
4. Discussion
- Results of the present study indicated that carbimazole affected the histological structure of prostate in albino rats. The prostate showed degeneration of epithelial cells, cytoplasmic vacuolization, hyperplasia and congestion of blood vessels. The effect of antithyroid drugs including carbimazole on reproductive system was studied in different animals. Anguiano et al.[22] reported that the enzymatic activity in epididymis, semen and prostate was completely inhibited by 6-n-propyl-2-thiouracil suggesting that local generation of T3 could be associated with the development and function of epididymis and spermatozoa maturation. Marty et al.[23] found that absolute testes, epididymal, prostate and seminal vesicle weights were decreased by 6-propylthiouracil. Also, it significantly decreased serum T4 level. Maran and Aruldhas[24] reported that daily administration of 0.05% methimazole to the nursing mothers induced many changes in newborn male rats; significantly reduced seminiferous tubules diameter, the proliferation and differentiation of germ cells were arrested and their number were decreased.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML