-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Intelligent Systems
p-ISSN: 2165-8978 e-ISSN: 2165-8994
2017; 7(4): 107-112
doi:10.5923/j.ajis.20170704.01

A Robustness Segmentation Approach for Skin Cancer Image Detection Based on an Adaptive Automatic Thresholding Technique
Razi J. Al-azawi1, Abbas Abdulazez Abdulhameed2, Hussein Majeed Ahmed3
1Department of Laser and Optoelectronics, University of Technology, Baghdad, Iraq
2Department of Computer Science, University of Mustansiriyah, Baghdad, Iraq
3Department of Computer Science, Iraqi Commission for Computers and Informatics/ Informatics Institute for Postgraduate Studies, Baghdad, Iraq
Correspondence to: Hussein Majeed Ahmed, Department of Computer Science, Iraqi Commission for Computers and Informatics/ Informatics Institute for Postgraduate Studies, Baghdad, Iraq.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Two type of automatic techniques are suggested and assessed and compared to the division of skin lesions in dermoscopic images. That includes modern automatic methods which have been effectively utilized in numerous therapeutic imaging issues. Success or disappointment computer-aided diagnosis systems dependent on the division step thoroughness. Used two types of methods in this work to the automatic division include the k-means and automatic thresholding. Utilizing a dataset of 200 images as ground truth manually sectioned by the specialist dermatologist to assessed the segmentation methods with four different metrics. Through executed division methods, the automatic thresholding technique accomplished the best division execution.
Keywords: Image segmentation, Automatic thresholding, Melanoma, Skin, Dermoscopy
Cite this paper: Razi J. Al-azawi, Abbas Abdulazez Abdulhameed, Hussein Majeed Ahmed, A Robustness Segmentation Approach for Skin Cancer Image Detection Based on an Adaptive Automatic Thresholding Technique, American Journal of Intelligent Systems, Vol. 7 No. 4, 2017, pp. 107-112. doi: 10.5923/j.ajis.20170704.01.
Article Outline
1. Introduction
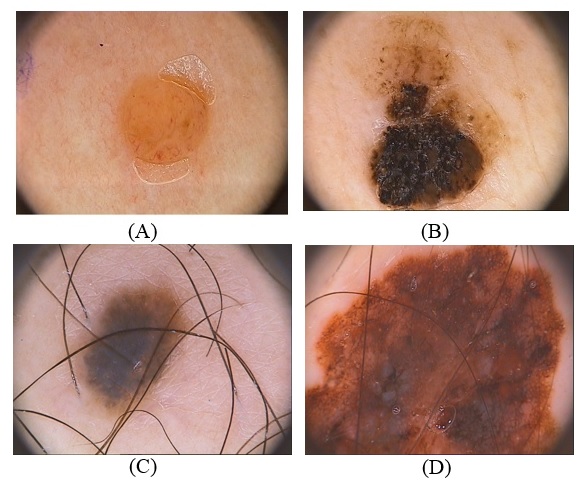
- One of the most common cancers in humans is skin cancer, the most deadly form of skin cancer is malignant melanoma, and it has been rapidly growing over the last years, the early diagnosis is very useful for successful treatment of melanoma, because when detected in early step the melanoma can surgically be removed with a fantastic diagnosis [1, 2]. The clinical detection of melanoma usually depends on the four principles are Asymmetric; Border anomaly; Color, and distance, or the seven points checklist, it is evaluation method for a set of various features relying upon colors, shapes, and textures [3]. Dermoscopy is the more developed technique to medical detection for a skin lesion as shown in (Fig.1), lesion analysis with an assist from a dermoscopy which amplifies the lesion from 5x up to 100x, enables the perception of their morphological structure [4]. there is actually a large attention in the evolution of (CAD) system that can help a clinical assessment of dermatologists, typical steps in automatic dermoscopy image analyses has generally four steps (a) hair removal and smoothing, (b) images segmentations, (c) features extractions and features selections, (d) classifications [7]. Segmentations steps are significant steps since its effects on an accuracy the following steps, therefore, the large variety of lesion shape, size, and color with various skin kinds, textures, and some lesions has an irregular boundary and in some status, a smooth transmission between the Lesions and the skin and hairs cover the lesions and the presence of shining reflection, all these reasons lead to difficult the segmentation [5]. Several algorithms have been suggested to address this problem, they can be broadly classified as adaptive thresholding, edge based or region-based methods, the good contrast between the lesion and the surrounding skin lead to achieving good results in adaptive thresholding methods [6].
2. Background and Motivation
- Skin tumor is a usually happening threat in fair-skinned people. The numeral of the skin tumor treatments growing robustly in the last decade and the cost of skin tumor was most elevated of all tumors in the United States [7-9]. There were around 76,250 new instances of melanoma and roughly 8,790 new melanoma-related death in 2012 in the United States [10]. Despite the occurrence average of melanoma in Asian are lower than in Caucasian, benign skin cancer, for example, squamous cell carcinomas (SCC) or basal cell carcinomas (BCC), participate to important morbidity among fair-skinned Asians [11]. In a modern estimate by the Australian government, the aggregate cost of diagnoses and treatments benign skin cancer was 511 million Australian dollars in 2010 and will be 703 million in 2015 [12].
3. Dataset
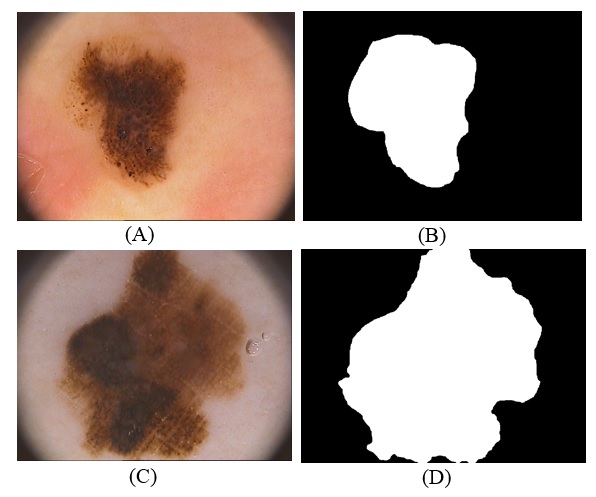
- In this study, we will have used Hospital Pedro Hispano (PH²) dataset. This images in dataset contain 200 dermoscopy images of lesions, it includes 80 joint nevi; 80 irregular nevi, and 40 malignant, it included medical annotations for all the images namely medical segmentations of the lesions, clinical and the histological diagnose and estimation of several dermoscopy criteria such as blue-whitish veils, regression area, dots/globules, streaks, pigment network, colors as shown in (Fig.1), it has the manual division for each image to be utilized as ground truth in the assessment of the division methods was accomplished by a specialist dermatologist as shown in (Fig.2.) [21].
4. Related Work
- “Different kinds of algorithms for the automatic segmentation of skin lesions in dermoscopic images were executed and evaluated, as mean-shift, automatic thresholding, region growing, gradient vector flow (GVF), k-means, and watershed, evaluated this method with three distinct metrics by using ground truth a database of 50 images manually segmented by an expert dermatologist, Among the executed segmentation approaches, the GVF snake method achieved the best segmentation performance” [13].“Six methods are Propose and evaluate for the segmentation of skin lesions in dermoscopic images, includes (gradient vector flow (GVF) and the level set method of Chan et al. [(C-LS)]. It also includes a set of methods developed by the authors which were tailored to this particular application (adaptive thresholding (AT), adaptive snake (AS), EMlevel set [(EM-LS), and fuzzy-based split and- merge algorithm (FBSM)], evaluated this method with four different metrics by applied to 100 dermoscopic images obtained from an experienced dermatologist as the ground truth, Among the executed segmentation approaches, the AS and EM-LS is achieved the better segmentation performance, which is semi-supervised methods. The best fully automatic method was FBSM, with results only slightly worse than AS and EM-LS” [14].“Evaluates and analyses the segmentation algorithms over dermoscopic images, such as region-based techniques (K-means, Fuzzy C-means, Expectation Maximization and Statistical Region Merging), thresholding techniques (Global and Adaptive), Contour models (Active Contour Model and Chan - Vese Model), the metrics that used to evaluate various segmentation methods are Accuracy, sensitivity, specificity, Border error, Hammoud distance, Hausdorff distance, MSE, PSNR and elapsed time that applied on the database of 20 images, all of the results display the fuzzy clustering, as well as Spectral Clustering algorithms, gives better results for dermoscopic images” [15].
5. Automatic Segmentation Methods
- Image segmentation is a wide and active field, not only for computer vision and satellite images but also for medical images, its aim is partitioning images into an area which are meant for a specific task, different technique and approaches are utilized, choosing a specific technique depend on the feature of the issue to be solved, Segmentation is the main step before the descriptions, recognitions or classifications of images or its constituents, There are two main methods; a) regions-based technique, in which likeness is discovered; b) boundaries-based technique, in which edge are discovered and linked to shape boundaries about areas, during the segmentation is important to use much relevant a priori information to develop strong interpretation systems, the Segmentation is the division of images into meaningful areas, commonly to differentiate objects or areas of attention (“foreground”) from everything other (“background”), In the easiest situations, there would be only this two portion ("foreground & background") and a segmentation image will be a binary image [16]. Segmentation is one of the difficult tasks in digital image processing, its technique is employed to separate the lesion pigment from the Proper skin, and dermoscopy image is a large defiance for segmentation algorithm because of the different diversities in skin and lesion [14]. Some images have low disparity or smooth transmission between the lesion and the surrounding skin, they commonly contain some skin feature like black frame; air bubbles; hairs; blood vessel and skin lines, a large number of divide algorithms have been proposed to defeat these challenges [17]. In this paper, we propose and compare two divide methods are, executed, and evaluated, including adaptive Automatic Thresholding and k-means.
5.1. Adaptive Automatic Thresholding
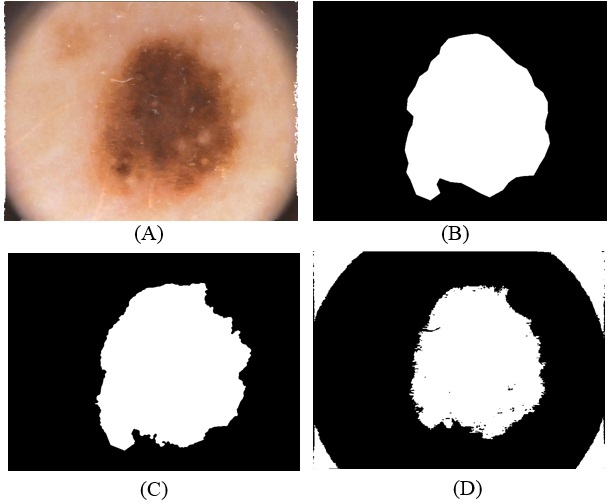
- Is One of the simplest segmentation methods is thresholding, it’s based on similarity approach and used for melanoma detection, it can be partitioned to two kinds’ global threshold and adaptive (local) threshold, Global Threshold is using a single thresholding value for the whole image, but in adaptive (local) threshold the image is divided into small images and then apply thresholding for each sub-image, in this case, The output is a binary image displaying the segmented part [18]. The advantages of thresholding method are, firstly it does well for images that have good contrast between background and foreground but not for images that have sharp peaks, secondly there is no need for any prior knowledge about the image, but this method fails because of the overlapping of two regions of the images [19].The adaptive image segmentation using thresholding is one of two method used in proposed method. It consists of a sequence of steps to segment image and at last region of interest. At first, convert skin image to grayscale image and then enhanced gray level contrast by using image adjustment that uses to maps some percentage of intensity values in the grayscale image to new values of low and high intensities. This increases the contrast of the skin image. These images will convert to binary. The important and sensitive case in converting a grayscale image to binary is threshold level. The binary image will invert to make the white area related to ROI. The segmentation starts with specifying the center of the region as a start point and do segmentation tracing until cover the whole of the boundary of the tumor. These operations will make boundary matrix that will fill inside when the tumor is located. After this operation is done the enhanced of segmentation is applied on the result by comparing it with GT image of the same image get it from the dataset. The comparison applied on each pixel if it is one on proposed segmentation or in GT image the result will be one.
5.2. K-Means Clustering
- Is a method that divides the image into multiple clusters, depending upon some distance metrics between cluster centroid and whole pixels, the goal of it is to divide the image into alternately exclusive clusters [20]. Each cluster is appeared by one prototype object, and a new data sample is marked to the closest prototype and then to the cluster, the training contains a simple repeat planner to set placing of prototypes; 1) At random choose k objects from the training set, which become the prototypes; 2) appoint all the other objects to the nearest prototype; 3) calculate the new prototype of class as the average of all topics which have the same label; 4) If the prototype have varied Dramatically, return to step 2 [16]. In this paper, most images that used having three distinguished areas identical to the lesions of the skin, and to the dark areas in all corners of the images, yet, a few images not show the dark areas in all corners and consist of two special objects, yet, in K-Means algorithm the cluster’s number can be either two or three, the clusters number is recognized automatic depend on the corner mask that is calculated in pre-processing stage [13].
6. Proposed Method
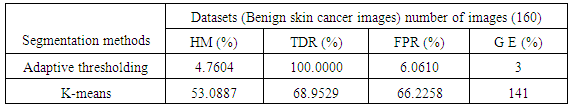
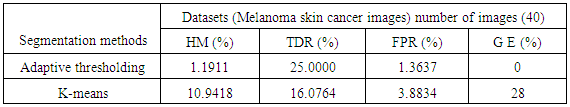
- A proposed method in this paper depend on four categories to compute the difference between each clustering methods and the Ground truth lesion which are Hammoude distance (HM), True Detection Rate (TDR), False Positive Rate (FPR), and gross - error [15].
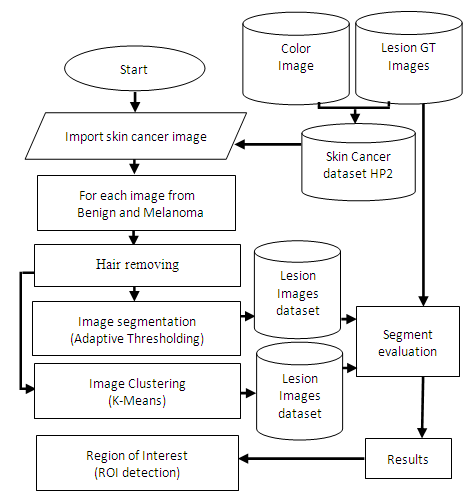 | Figure 4. General view of proposed method |
6.1. Region of Interest Detection
- The region of interest detection based in proposed method depend on two method of segmentation as explain before. The region of interest used later for localization feature extraction. Skin cancer clustering depend on four category to compute the differences between each clustering (segmentation method and the ground truth lesion GT (binary image) union one: Hammoude Distance (HM), True Detection Rate (TDR), False Position Rate (FPR), and Cross error. These will find using equation [1-4] respectively. The result of these four parameter use for evaluate the result of segmentation in each method used in proposed method. The best will choose to find ROI and later be input to feature extraction step. The ROI is sensitive step because of the total feature depend on it, especially structural features are depend on the extracted binary image.
7. Experimental Result
- The executed segmentation technique was an assessment on a group of 200 images acquired from PH² dataset, the manual division of each image to be utilized as ground truth (GT) in the assessment of the segmentation technique was done by a specialist dermatologist. The previous studies [13, 14, 15] used the same dataset but the number of images is (50, 100, 20) respectively. Four performance measurements were utilized to evaluate the limit different, namely Hammoude distance (HM), True detection rate (TDR), false positive rate (FPR), and Gross error.#HM compares a pixel by pixel of the pixels enclosed by the two border:
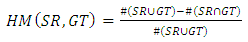 | (1) |
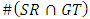 The number of white pixels in the common areas only,
The number of white pixels in the common areas only,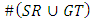 The number of white pixels in the white areas of both images.#TDR computes the average of pixels that categorized as lesions by both the medical expert and the automatic segmentations:
The number of white pixels in the white areas of both images.#TDR computes the average of pixels that categorized as lesions by both the medical expert and the automatic segmentations: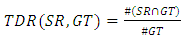 | (2) |
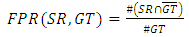 | (3) |
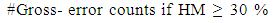 | (4) |
|
|
8. Conclusions
- In this paper, two algorithms for automatic segmentation of dermoscopy image were executed and assessed for tracking the boundary of skin cancer lesions, including the Adaptive automatic thresholding, k-means clustering. The result of each algorithm is greatly influenced by the type of images used for analysis. Among the implemented algorithms the Adaptive automatic thresholding technique accomplished the better results and confirm to be useful and firm enough to the automatically skin lesions segmentations in computer-aided diagnosis systems.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML