-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(1): 25-27
doi: 10.5923/j.ajis.20120201.05
A New Insight on the Synthesis of 2,4,5-Triaryl-1H-imidazoles in the Absence of Catalyst
Reza Tayebee , Malihe Ghadamgahi
Department of Chemistry, School of Sciences, Sabzevar Tarbiat Moallem University, Sabzevar, 96179-76487, Iran
Correspondence to: Reza Tayebee , Department of Chemistry, School of Sciences, Sabzevar Tarbiat Moallem University, Sabzevar, 96179-76487, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The new results concerning synthesis of some 2,4,5-triaryl-1H-imidazoles in the absence of any additive, as catalyst, is presented. Moreover, we modified the experimental route for the isolation and purification of the un-reacted benzyl, as initial reactant, from products at the end of the reaction.
Keywords: Tri-aryl-imidazole, Multi-component, Synthesis, Green
Cite this paper: Reza Tayebee , Malihe Ghadamgahi , "A New Insight on the Synthesis of 2,4,5-Triaryl-1H-imidazoles in the Absence of Catalyst", American Journal of Organic Chemistry, Vol. 2 No. 1, 2012, pp. 25-27. doi: 10.5923/j.ajis.20120201.05.
1. Introduction
- Imidazole containing compounds have widespread applications in organic synthesis and in pharmaceutical research. Substituted imidazoles are known as inhibitors of P38MAP kinase[1], fungicides and herbicides[2], plant growth regulators[3], and therapeutic agents[4]. Furthermore, they are of interest due to their herbicidal, analgesic, fungicidal, anti-inflammatory, and antithrombotic activities[5]. There are several methods in the literature for the synthesis of 2,4,5-triaryl-1H-imidazoles from benzil/benzoin, aldehydes and ammonium acetate using different catalyst such as zeolite HY/silica gel[6], ZrCl4[7], NiCl2.6H2O[8], iodine [9], sodium bisulfite[10], acetic acid[11], and NH4OAc[12] (Scheme 1). However, these methods require prolonged reaction time, exotic reaction condition and high cost of catalysts and. Therefore, development of new strategies for the preparation of 2,4,5-triaryl-1H-imidazole derivatives would be highly desirable. The art of performing efficient chemical transformation coupling three or more components in a single operation by a catalytic process avoiding stoichiometric toxic reagents large amounts of solvents and expensive purification techniques represents a fundamental target of the modern organic synthesis. Accordingly, multi-component condensation reactions provided an especially attractive synthesis method for fast and effective generation of products. The use of solid acid catalysts[13] has attracted a vast importance in organic synthesis due to their several advantages including operationally simplicity, no toxicity, reusability, low cost, and ease of isolation after completion of the reaction.Potassium dihydrogen phosphate (KH2PO4) as a buffer,neutralizing agent, and yeast food also applied as an efficient heterogeneous acid catalyst[14,15]. Potassium dihydrogen phosphate has been found as a mild and effective catalyst in synthesis of 2,4,5-Triaryl-1H -imidazoles under reflux. Preparation of 2,4,5-triaryl-1H-imidazoles needs at least 2 mole of ammonium acetate against each mole of benzyl. However, reported catalytic procedures used 4-8 mole of NH4OAc per each mole of diketone or benzil. Recently, we found that this reaction is mainly catalyzed by >8 mol ratio of NH4OAc and led to >70% of the corresponding imidazoles.
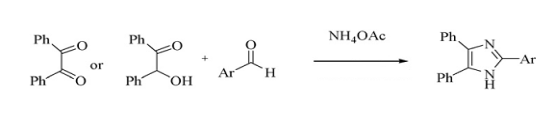 | (Scheme 1). |
2. Experimental
- All starting materials were purchased commercially and were used as received. All products were characterized by comparison of their spectral and physical data with those reported in the literature. Silica gel 60 (70—230 mesh) was used for column chromatography. Progress of the reactions was monitored by TLC. Infrared spectra were recorded (KBr pellets) on a 8700 Shimadzu Fourier Transform spectrophotometer. 1HNMR spectra were recorded on a Bruker AVANCE 300-MHz instrument. A mixture of benzaldehyde (10 mmol), benzyl (10 mmol), ammonium acetate (20 mmol), were refluxed with stirring in ethanol for 40 min. The mixture was cooled and cold water was added and the residue washed with hot petroleum benzen to afford the pure product. The pure product, if needed, could be obtained by re-crystallization from ethanol-water mixture. All products were identified by means of IR and 1H NMR spectroscopy and/or comparison of their melting points with those reported in the literature.
3. Results and Discussion
- In continuation of our research program on the use of simple inorganic non-toxic catalysts, we report herein the efficacy of KH2PO4 as catalyst. In this study the multi- component reaction strategy for the synthesis of 2,4,5-triaryl-1H-imidazole by using benzil/benzoin, various substituted aldehydes and ammonium acetate in presence of KH2PO4 as catalyst, in ethanol at reflux condition is introduced.Preparation of the title compound has been reported with different amounts of ammonium acetate. For example in the synthesis of this compound with ZrOCl2.8H2O and Sodium Bisulfite, the mol ratio of benzealdehyde, benzyl and ammonium acetate has been 2.4:2:8 and with Phosphomolybdic acid 2.4:2:6[4,5]. Table 1 shows some catalytic systems using different amounts of NH4OAc.
|
|
4. Conclusions
- In conclusion, this report illustrated the new findings on the synthesis of some 2,4,5-triaryl-1H-imidazoles in the absence of any additive as catalyst. Moreover, the modified experimental route for the isolation and purification of the un-reacted benzyl, as initial reactant, from products at the end of the reaction was studied. 这里因为没有使用正确的样式漏掉了,样式名称为 SAP27-ReferenceItem
References
| [1] | J. C. Lee, J. T. Laydon, P. C. Mcdonnel, T. F. Gallagher, S. Kumar, D. Green, D. Mcnulty, M. J. Blumenthal, J. R. Keys, S. W. Land Vatter, J. E. Strickler, M. M. Mclaughlin, I. R. Siemens, S. M. Fisher, G. P. Livil, J. R. White, and J. L. Adams, 1994, A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739 |
| [2] | T. Maier, R. Schmierer, K. Bauer, and et al., US Patent 4820335, 1989 |
| [3] | R. Schmierer, H. Mildenberger, and H. Buerstell, German Patent, 361464, 1988, Chem Abstr 108: 37838 |
| [4] | J. Heeres, L. J. J. Backx, J. H. Mostmans, and J. Van Cutsem, 1979, . J. Med. Chem. 22, 1003 |
| [5] | S. Balalaie, A. Arabanian, and M.S. Hashtroudi, 2000, . Mont. Fur. Chem. 131, 945 |
| [6] | G. V. M. Sharma, Y. Jyothi, and P. S. Lakshmi, 2006, Efficient Room‐Temperature Synthesis of Tri‐ and Tetrasubstituted Imidazoles Catalyzed by ZrCl4. Synth. Commun. 36, 2991 |
| [7] | M. M. Heravi, K. Bakhtiari, H. A. Oskooie, and S. Taheri 2007, Synthesis of 2,4,5-triaryl-imidazoles catalyzed by NiCl2·6H2O under heterogeneous system. J. Mol. Catal. A Chem. 263, 279 |
| [8] | , , and , 2006, . Mont. Fur. Chem. 137, 1189 |
| [9] | , , and , 2008, . Mont. Fur. Chem. 139, 125 |
| [10] | S. E. Wolkenberg, D. D. Wisnoski, W. H. Leister, Y. Wang, Z. Zhao, and C. W. Lindsley, 2004, . Org. Lett. 6, 1453 |
| [11] | M. Kidwai, S. Saxena, and S. Rastogi, 2005, An Efficient Synthesis of 2,4,5-Trisubstituted and1,2,4,5-Tetrasubstituted-1H-imidazoles. Bull. Korean Chem. Soc. 26, 2051 |
| [12] | J.H. Clark, 2002, Acc. Chem. Res. 35, 791 |
| [13] | A. Saikia, M. G. Barthakur, M. Borthakur, C. J. Saikia, U. Bora, R. C. Boruah, 2006, . Tetrahedron Lett. 47, 43 |
| [14] | F. Xu, H.X. Lv, J.P. Wang, et al., 2008, J. Chem. Res. 12 (4), 707 |
| [15] | R. S. Joshi,, P. G. Mandhan, M. U. Shaikh, R. P. Kale, and C. H. Gill, 2010, Potassium dihydrogen phosphate catalyzed one-pot synthesis of 2,4,5-triaryl-1H-imidazoles. Chin. Chem. Lett. 21, 429 |
| [16] | Z. Karimi-Jaberi, and M. Barekat, 2010, One-pot synthesis of tri- and tetra-substituted imidazoles using sodium dihydrogen phosphate under solvent-free conditions. Chin. Chem. Lett. 21, 1183 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML