E. A. Gomaa, B. M. Al-Jahdali
Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt
Correspondence to: E. A. Gomaa, Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Abtract New equation was applied for the Calculation of association constant (KA) from the reaction of Cu(NO3)2 with Kryptofix-221 in mixed (MeOH-DMF) and in absence and Presence of ligand at different temperatures. From the experimental results, the molar conductance (Λ) were evaluated. The limiting molar conductance (Λο) were also estimated. Gibbs free energies of association (ΔGA) was also estimated, Moreover, recalculation of (KA) was achieved by applying Shedlovsky and Fouss-Kraus extrapolation methods. In addition, the molar solvated (V), Van der Waals (VW), electrostriction (Ve) and apparent molar (Øv) volmes were calculated. Also, the enthalpy change (ΔH) and the entropy change (TΔS) for Cu(NO3)2 were calculated. The degree of dissociation (α) were also calculated. All the results were discussed in view of ion-solvent interactions.
Keywords:
Association, Kryptofix-221-Dissociation, Molar Solvated Volume, Van der Waals Volume, Electrostriction Volume, Free Energy of Association, Denisties, Molar Fraction, Limiting Conductance
Cite this paper: E. A. Gomaa, B. M. Al-Jahdali, Association of Cu(NO3)2 with Kryptofix-221 in Mixed (MeOH-DMF) Solvents at Different Temperatures, American Journal of Fluid Dynamics, Vol. 1 No. 1, 2011, pp. 4-8. doi: 10.5923/j.ajfd.20110101.02.
1. Introduction
Although metal cations play an important role both in chemistry and biology, the coordination chemistry of metals was completely ignored by chemists. However, the coordination chemistry of metal cations has mainly developed by the synthesis of crowns by Pedersen[Pedersen, (1967)][1]. The discovery of the crown ethers was followed by synthesis of macro bicyclic poly ethers containing three poly ether strands joined by two bridge head nitrogens[Lehn et al., (1971)][2].These compounds have three-dimentional cavites which can accommodate a metal ion of a suitable size and from an inclusion complex.These ligands which developed by Lehn and his– coworkers [Dietrich et al., (1969)][3], were called[2] – Cryptands where[2] indicates the bicyclic ligand such as Kryptofix-221 which its structure is given in Fig (1). The crown compounds and their thia – and aza – derivatives have a considerable interest in terms of their complexation properties in solution with univalent and bivalent metals[Gokel, (1976)][4]. It is important to mention that the marocyclic crown ethers have many applications[Izatt et al., (1978)].[5] in biological activity, corrosion chemistry, analytical chemistry, phase – transfer catalysis and industrial production such as nuclear energy, electronics and electro–chemical photosensitive materials[El-Dossouki; (1998)][6].On understanding the interactions between macrocyclic crown ethers such as Kryptofix-221 and metal cations in solutions, it requires the study of various parameters govering these interactions. The thermodynamic studies of these interactions gave important informations about their complexation reactions and the selectivities of these ligands towards different metal cations[Rounaghi et al., (1999)][7]. The abserved association constant values are known to be a composite quantities depending on specific and non-specific solute – solvent interactions. The separation of various interaction contributions is often very difficult process, beside that using mixed solvents it add another dimention to the problem[Mukhopadhyay et al., (1997)][8]. The multidentate macro molecules (MMM) which have been studied as ligands for Mz+ were included natural antibiotics and synthetic compounds such as crowns and cyptands[9]. The macro molecular ligands had recently become more important to the chemistry of Mz+ than the conventional ligands, this was because they binded Mz+effectively and rendered the latter soluble in non-polar solvents and because they were more relevant to the chemisrtry of Mz+in biological system which thereinvolved essentially the macrobiomolecules.A conductance study of the interaction between Co2+, Ni2+, Cu2+, Cd2+, Zn2+ and pb2+ ions with Kryptofix-221, K-22& K-222 in different (acetonitrile-dimethyl sulfoxide) mixtures was carried out at various temperatures by Shamsipur[10]. The aim of the present work is to study the conductivity of Cu(NO3)2 in the absence and in presence of Kryptofix-221 using different molar ratios of (MeOH-DMF) mixed solvents at different temperaturas. By applying Shedlovsky,Fouss- Kraus extrapolations[11] methods, we were able to evaluate the values of (Λο), (KA), (ΔGA) and to make an acceptable discussion.Finally, the crystal and molecular structures of about 200 metal halide complexes with oxygen–Containing crown ethers were investigated by Bel'sky[12].The characteristic features of the formations of these complexes and their coordination fragments were discussed.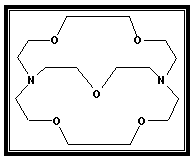 | Figure 1. Kryptofix- 221 [4,7,13,16,21- pentaoxa-1.10-diazo-bicyclo [8,8,5] tricosane] |
2. Experimental
The aza-crown ether, Kryptofix-221[4.7.13.16.21-pantaoxa - 1.10 – diazo bicyclo[8.8.5] tricosane) was supplied from Merck Co. where as, cupper nitrate Cu(NO3)2 of high grade was supplied from BDH and it was used without any further purification.The water content of the salt was determined by using (Mettler Dl-18) Karl-Fisher titrator and it was found to be less than ± 0.01%.All solvents used MeOH&DMF were BDH supplements used without any further purification.The measurements of the specific conductivity of Cu(NO3)2 in the presence of Kryptofix-221 in all the mixed (MeOH - DMF) solvents were achieved at different temperatures using Beckman Conductivity Bridge Model No. (RE – 18A).All the conductometric titrations were done using 1xl0-3 mol./lit. Cu (NO3)2 and 1xl0-4 mol. /lit. of 332, 44 gm/mol, Kryptofix-221.Spectrophotometrical contineous variation study of Cu(NO3)2 in the presence of Kryptofix-221 at different temperatures and in 20% MeOH was achieved using Unicam UV-2-100 UV/Visible spectrometer v 3.32; at wave length of λ max (284nm).
3. Results and Discussion
The specific conductance values (Ks) of different concentrations of Cu(NO3)2 in (MeOH-DMF) mixtures in the absence and in the presence of Kryptofix-221, were measured experimently and from which the values of molar conductance (Λ) were calculated [Walter, (1976)] by using eq. (1):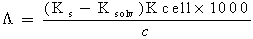 | (1) |
Where (Ks) and (Ksolv) are the specific conductances of the solution and the solvent, respectively; (Kcell) is the cell constant and (C) is the molar concentration of Cu (NO3)2.The association constant value (KA) of different concentrations of Cu (NO3)2 in (MeOH - DMF) mixtures in the presence and in the absence of Kryptofix-221, were calculated by using eq.(2)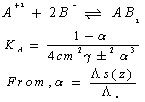 Where (Λ) is the molar conductance, (Λ o) is the limiting molar conductance.
Where (Λ) is the molar conductance, (Λ o) is the limiting molar conductance.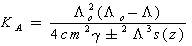 | (2) |
Where (α) is the degree of dissociation, Fuoss-Shedlovsky parameters (S, Z and s(z), activity coefficient (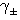 ) association constant (KA).The evaluations of Gibbs free energies of association (ΔGA) for Cu(NO3)2 with Kryptofix-221 in (MeOH -DMF) mixtures at different temperatures, were gained according to eq.(3).[Kappenstein, (1974) ][13].
) association constant (KA).The evaluations of Gibbs free energies of association (ΔGA) for Cu(NO3)2 with Kryptofix-221 in (MeOH -DMF) mixtures at different temperatures, were gained according to eq.(3).[Kappenstein, (1974) ][13]. | (3) |
From the densities measurments of solvents (MeOH-DMF) and the densities of Cu (NO3−)2 at different temperatures, the molar volumes (V) were calculated and their values are listed in Table (1). The packing density (P) as reported by Kim and Gomaa[14], i.e, the relation between Vander Waals volume (VW) and the molar volume (V) as shown in the following eq. (4).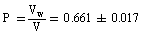 | (4) |
The electrostrication volume (Ve), which is the volume compressed by the solvent can be calculated by using eq (5) as following: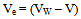 | (5) |
The apparent molar volumes 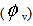 were also calculated by the following eq. (6)[15].
were also calculated by the following eq. (6)[15].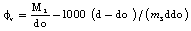 | (6) |
Where, (M2) is the M.Wt of DMF, (do) is the density of the solvent and (ms) is the molality .The values of the solvated radius (rs) were calculated by using eq. (7) [ (Gomaa, (16)] | (7) |
| Table 1. The molar volume (V), solvated Vander Waals(VW) and electrostriction volumes (Ve) of Cu(NO3)2 in (MeOH-DMF) mixtures at different temperatures |
| | Vol% MeoH | V(cm3.mol.-1) | VW(cm3.mol.-1) | Ve(cm3.mol.-1) | | | 298.15K | 303.15K | 313.15K | 313.15K | 298.15K | 303.15K | 308.15K | 313.15K | 298.15K | 303.15K | 308.15K | 313.15K | | O | 77.20 | 77.81 | 77.90 | 78.52 | 51.02 | 51.43 | 51.49 | 51.90 | -26.18 | -26.38 | -26.41 | -26.62 | | 20 | 65.42 | 65.91 | 65.99 | 66.33 | 43.24 | 43.56 | 43.61 | 43.84 | -22.18 | -22.35 | -22.38 | -22.49 | | 40 | 55.45 | 55.88 | 56.07 | 56.40 | 36.65 | 36.93 | 37.06 | 37.28 | -18.8 | -18.95 | -19.01 | -19.12 | | 100 | 40.76 | 41.16 | 41.26 | 41.47 | 26.94 | 27.20 | 27.27 | 27.41 | -13.02 | -13.96 | -13.99 | -14.06 |
|
|
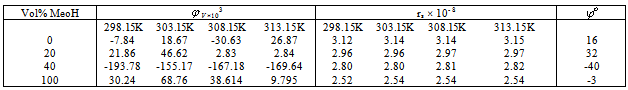
Table 2. The apparent molar volumes (
 ), the solvated radius(rs) and the apparent molar volume at infinite dilution ( ), the solvated radius(rs) and the apparent molar volume at infinite dilution (
 ) of Cu(NO3)2 in (MeOH – DMF) mixtures at different temperatures ) of Cu(NO3)2 in (MeOH – DMF) mixtures at different temperatures
 |
| |
|
Table 3. The values of C,
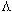 , ,
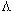 o, S(z), o, S(z),
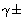 , ,
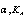 and and
 G of Cu(NO3)2 in mixed (MeOH-DMF) solvents at different temperatures using Fuoss – Shedlovesky method G of Cu(NO3)2 in mixed (MeOH-DMF) solvents at different temperatures using Fuoss – Shedlovesky method
 |
| |
|
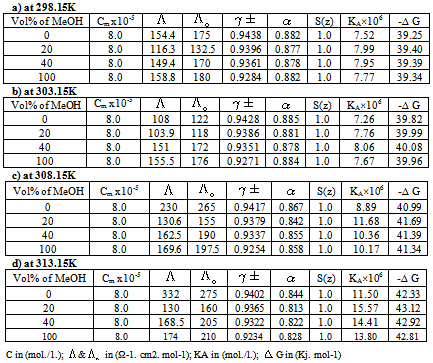
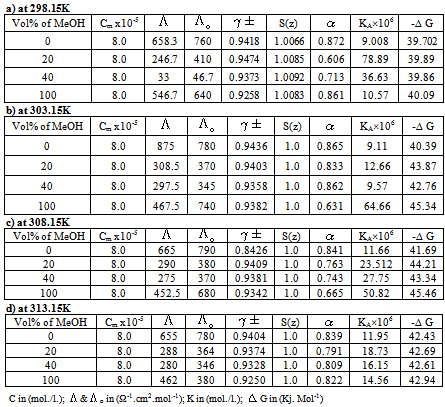
Table 4. The Values of C,
 , ,
 o, o,
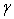 ±, S(z), ±, S(z),
 , KA and , KA and
 G of Cu(NO3)2 in presence of Kryptofix-221 and in (MeOH-DMF) mixtures, at different temperatures using Fuoss-Sheslovesky method G of Cu(NO3)2 in presence of Kryptofix-221 and in (MeOH-DMF) mixtures, at different temperatures using Fuoss-Sheslovesky method
 |
| |
|
Where (V) is the molar volume, (N) is Avogadro's number and (σ) is the diameter, the evaluated data are given in Table(2).The experimental condctometric data of the equivalent measurements of Cu(NO3)2 in mixed solvents were analyzed using Shedlovsky and Fuoss – Kraus extrapolation techniques which have been mentioned earlier in Dash's publication[17] as given.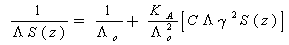 | (8) |
Where S(z) = 1 + Z + Z2 / 2 + Z3 / 3 + etc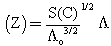 | (9) |
Where S = a 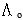 + b,a = 8.2 × 105 / (
+ b,a = 8.2 × 105 / (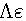 T)3/2, b =
T)3/2, b =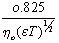 and
and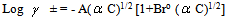 | (10) |
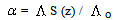 | (11) |
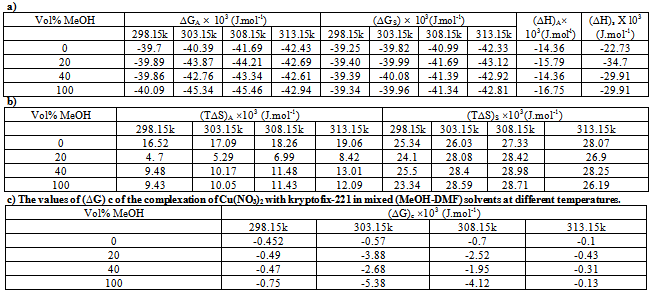
Where (A) and (B) are the Debye-Huckel constants, (ro) is the ion size parameter, (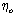 ) and (ε) are the viscosity and the dielectric constants of the MeOH-DMF mixed solvent, respectively. All the parameters calculated by Shedlovesky methode at different temperatures are given in Tables (3), (4).Table (5) (a,b,c), illustrated the thermodynamic parameters
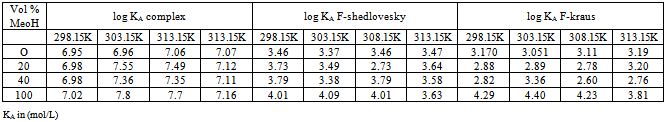
) and (ε) are the viscosity and the dielectric constants of the MeOH-DMF mixed solvent, respectively. All the parameters calculated by Shedlovesky methode at different temperatures are given in Tables (3), (4).Table (5) (a,b,c), illustrated the thermodynamic parameters 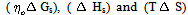 of the solvation of Cu(NO3)2 with (MeOH-DMF) in presence and absence of Kryptofix-221.All their values were calculated from the solubilites measurements. The (ΔGs) values found to be increased by increasing the content of the organic solvent (MeOH-DMF) mixtures.On the other hand, the values of (ΔH)s and (TΔS)s were generally found to be decreased by increasing the methanol content in the mixtures used.It was concluded from Table (6), that, the association constant (KA) of Cu(NO3)2 in presence and absence of Kryptofix-221 in (MeOH – DMF) mixtures, at different temperatures, using the different methods [Fuoss – Shedlovesky and Fuoss- Kraus] have nearly the same values. This indicates that, the association constants in this case are due to the formation of different stiochiometric complexes as mentioned in this table. The formation of these complexes are probably be outside the Kryptofix ring.The graphical presentation of the relation between log KA and the inverse temperatures
of the solvation of Cu(NO3)2 with (MeOH-DMF) in presence and absence of Kryptofix-221.All their values were calculated from the solubilites measurements. The (ΔGs) values found to be increased by increasing the content of the organic solvent (MeOH-DMF) mixtures.On the other hand, the values of (ΔH)s and (TΔS)s were generally found to be decreased by increasing the methanol content in the mixtures used.It was concluded from Table (6), that, the association constant (KA) of Cu(NO3)2 in presence and absence of Kryptofix-221 in (MeOH – DMF) mixtures, at different temperatures, using the different methods [Fuoss – Shedlovesky and Fuoss- Kraus] have nearly the same values. This indicates that, the association constants in this case are due to the formation of different stiochiometric complexes as mentioned in this table. The formation of these complexes are probably be outside the Kryptofix ring.The graphical presentation of the relation between log KA and the inverse temperatures 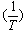 at different temperatures, give slope and from their slopes, we can calculate the thermodynamic parameters (ΔHA).Also, we plot the relation between log
at different temperatures, give slope and from their slopes, we can calculate the thermodynamic parameters (ΔHA).Also, we plot the relation between log 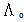 and
and 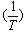 in presence and absence of Kryptofix-221.The graphical presentation of the relation between the association free energies (ΔGA) and the mole fraction (Xs) of the organic solvent used at different temperatures at four different mole fraction of Cu(NO3)2 and Kryptofix-221 are show in .Also the graphical presentation of the relation between the apparent molar volume
in presence and absence of Kryptofix-221.The graphical presentation of the relation between the association free energies (ΔGA) and the mole fraction (Xs) of the organic solvent used at different temperatures at four different mole fraction of Cu(NO3)2 and Kryptofix-221 are show in .Also the graphical presentation of the relation between the apparent molar volume 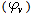 and (
and (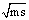 ), where (
), where (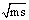 ) in the molality shown in .
) in the molality shown in .
4. Conclusions
It was concluded that, the KA association of metal cation with Kryptofix increase with increase of temperatures and also, with increase of the methanol content in the mixtures due to the increase of ion-ion and ion-solvent interactions. KA values increased by increase methanol content in the mixed solvents due to the preferential slovation of cupper with methanol than the mixed solvent and dimethyl formamide. KA values increase also by increasing temperatures due mainly to the increase in kinetic energy and kinetic work necessary to do salvation process. Log KA values calculated by different methods indicate that the values are small in case of Fuoss and Fuoss-Kraus methods in comparison to that values which have calculated. Therefore our values are near to the true picture of the greater ions. It was also concluded that the free energies of complexation increase with increase in methanol content due to the increase of interaction of the divalent metal cation with Kryptofix-221. According to the too low concentration used, the entropy parameter show no significant rule in this work.Table 5. The values of ΔG, ΔH and TΔS of Cu(NO3)2 in mixed (MeOH-DMF) solvents in presence and absence of Kryptofix-221 at different temperatures
 |
| |
|
Table 6. The values Log KA of Cu(NO3)2 in presence of Kryptofix -221 and in (MeOH-DMF) at different temperatures using different methods
 |
| |
|
References
| [1] | Pedersen C.J. (1967) Am. Chem. SoC.89.7017 |
| [2] | Lehn J.M.; Sauvage J.P. (1971) Chem. Comm., 440 |
| [3] | Dietrich B.; Lehn J.M. and Sauvage J.P. (1969) Tetrahedran Lett., 2885& 2889 |
| [4] | Gokel G. W.; Durst H.D. (1976) Synthesis, 168 |
| [5] | Izatt R.M.; Izatt N.E.; Rossiter B.E.; Christensen J.J. and Haymore B.L. (1978) Science 199,994 |
| [6] | El-Dossouki F.I. (1998) Ph.D. Thesis; Mansoura University; Egypt |
| [7] | Rounaghi G.; Nejad F.M. and Taheri K. (1999) Ind. J.Chem. 38A, 568 |
| [8] | Mukhopadhyay A.; Chattopadhyay M.R. and Pal M. (1997) Ind. J.Chem. 36A, 94 |
| [9] | Poonia N.S.; Bajai A.V, Coordination Chemistry of Alkaline and Alkali Earth Cations, Chem. Rev, 79. (1979), 389 |
| [10] | Shamsipur M.; Ghasem; J, J Inclusion Phenom Mol Recognit Chem, 20(2), (1995), 157 |
| [11] | Fouss R.M.; Edelson D. (1951) J.Am.Chem.Soc.73,269 |
| [12] | Bel'sky K.V., Chem Rev, 68(2), (1999). 119 |
| [13] | Kappenstein C. (1974) Bull. Soc.Chim. Fr. 89,101 |
| [14] | Kim J.I, Zeitschrift Fur Physikalische Chemie Neue Folge, 110, (1978), 197and Gomaa E.A.Thermochim.Acta ,152 (198) 371 |
| [15] | Gryblavwski W.; Pastewslai Electro Chimice Acta, 25(1980) 279 |
| [16] | Gomaa E.A., Therm.Chim. Acta, 152 (1989) 71 |
| [17] | Dash N.U; Pasupalak N.N, Ind. J.Chem, 36(A), (1997) 88 |


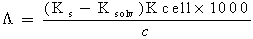
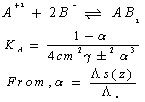 Where (Λ) is the molar conductance, (Λ o) is the limiting molar conductance.
Where (Λ) is the molar conductance, (Λ o) is the limiting molar conductance.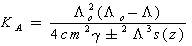
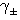 ) association constant (KA).The evaluations of Gibbs free energies of association (ΔGA) for Cu(NO3)2 with Kryptofix-221 in (MeOH -DMF) mixtures at different temperatures, were gained according to eq.(3).[Kappenstein, (1974) ][13].
) association constant (KA).The evaluations of Gibbs free energies of association (ΔGA) for Cu(NO3)2 with Kryptofix-221 in (MeOH -DMF) mixtures at different temperatures, were gained according to eq.(3).[Kappenstein, (1974) ][13].
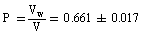
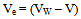
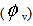 were also calculated by the following eq. (6)[15].
were also calculated by the following eq. (6)[15].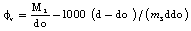

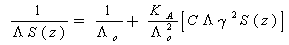
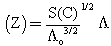
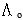 + b,a = 8.2 × 105 / (
+ b,a = 8.2 × 105 / (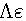 T)3/2, b =
T)3/2, b =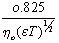 and
and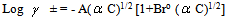
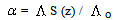
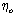 ) and (ε) are the viscosity and the dielectric constants of the MeOH-DMF mixed solvent, respectively. All the parameters calculated by Shedlovesky methode at different temperatures are given in Tables (3), (4).Table (5) (a,b,c), illustrated the thermodynamic parameters
) and (ε) are the viscosity and the dielectric constants of the MeOH-DMF mixed solvent, respectively. All the parameters calculated by Shedlovesky methode at different temperatures are given in Tables (3), (4).Table (5) (a,b,c), illustrated the thermodynamic parameters 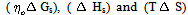 of the solvation of Cu(NO3)2 with (MeOH-DMF) in presence and absence of Kryptofix-221.All their values were calculated from the solubilites measurements. The (ΔGs) values found to be increased by increasing the content of the organic solvent (MeOH-DMF) mixtures.On the other hand, the values of (ΔH)s and (TΔS)s were generally found to be decreased by increasing the methanol content in the mixtures used.It was concluded from Table (6), that, the association constant (KA) of Cu(NO3)2 in presence and absence of Kryptofix-221 in (MeOH – DMF) mixtures, at different temperatures, using the different methods [Fuoss – Shedlovesky and Fuoss- Kraus] have nearly the same values. This indicates that, the association constants in this case are due to the formation of different stiochiometric complexes as mentioned in this table. The formation of these complexes are probably be outside the Kryptofix ring.The graphical presentation of the relation between log KA and the inverse temperatures
of the solvation of Cu(NO3)2 with (MeOH-DMF) in presence and absence of Kryptofix-221.All their values were calculated from the solubilites measurements. The (ΔGs) values found to be increased by increasing the content of the organic solvent (MeOH-DMF) mixtures.On the other hand, the values of (ΔH)s and (TΔS)s were generally found to be decreased by increasing the methanol content in the mixtures used.It was concluded from Table (6), that, the association constant (KA) of Cu(NO3)2 in presence and absence of Kryptofix-221 in (MeOH – DMF) mixtures, at different temperatures, using the different methods [Fuoss – Shedlovesky and Fuoss- Kraus] have nearly the same values. This indicates that, the association constants in this case are due to the formation of different stiochiometric complexes as mentioned in this table. The formation of these complexes are probably be outside the Kryptofix ring.The graphical presentation of the relation between log KA and the inverse temperatures 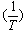 at different temperatures, give slope and from their slopes, we can calculate the thermodynamic parameters (ΔHA).Also, we plot the relation between log
at different temperatures, give slope and from their slopes, we can calculate the thermodynamic parameters (ΔHA).Also, we plot the relation between log  and
and 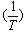 in presence and absence of Kryptofix-221.The graphical presentation of the relation between the association free energies (ΔGA) and the mole fraction (Xs) of the organic solvent used at different temperatures at four different mole fraction of Cu(NO3)2 and Kryptofix-221 are show in .Also the graphical presentation of the relation between the apparent molar volume
in presence and absence of Kryptofix-221.The graphical presentation of the relation between the association free energies (ΔGA) and the mole fraction (Xs) of the organic solvent used at different temperatures at four different mole fraction of Cu(NO3)2 and Kryptofix-221 are show in .Also the graphical presentation of the relation between the apparent molar volume 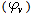 and (
and (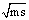 ), where (
), where (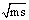 ) in the molality shown in .
) in the molality shown in . Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML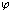 ), the solvated radius(rs) and the apparent molar volume at infinite dilution (
), the solvated radius(rs) and the apparent molar volume at infinite dilution (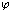 ) of Cu(NO3)2 in (MeOH – DMF) mixtures at different temperatures
) of Cu(NO3)2 in (MeOH – DMF) mixtures at different temperatures 
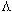 ,
, 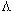 o, S(z),
o, S(z), 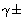 ,
, 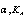 and
and 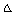 G of Cu(NO3)2 in mixed (MeOH-DMF) solvents at different temperatures using Fuoss – Shedlovesky method
G of Cu(NO3)2 in mixed (MeOH-DMF) solvents at different temperatures using Fuoss – Shedlovesky method 
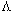 ,
,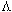 o,
o, 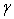 ±, S(z),
±, S(z),  , KA and
, KA and  G of Cu(NO3)2 in presence of Kryptofix-221 and in (MeOH-DMF) mixtures, at different temperatures using Fuoss-Sheslovesky method
G of Cu(NO3)2 in presence of Kryptofix-221 and in (MeOH-DMF) mixtures, at different temperatures using Fuoss-Sheslovesky method 

