-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2021; 11(1): 13-15
doi:10.5923/j.ajee.20211101.02
Received: Jun. 27, 2021; Accepted: Jul. 21, 2021; Published: Jul. 30, 2021

Accumulation of Selenium in Fathead Minnows (Pimrphales Promelas)
Shahrokh Ghaffari, Shadi Abu-Baker, Erin Glynn
Department of Chemistry and Biochemistry, Ohio University, Zanesville, Ohio, USA
Correspondence to: Shadi Abu-Baker, Department of Chemistry and Biochemistry, Ohio University, Zanesville, Ohio, USA.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The purpose of conducting this undergraduate research project is to test the amount of selenium absorbed by fathead minnows when selenium is added to the water supply. Selenium is a nonmetallic element that is found in a variety of chemical forms both in marine and freshwaters (3). In this study there were two groups of fathead minnows studied. One was the control and the other contained 20 ppm of selenium in selenite form. The fathead minnows were placed in a 25-degree Celsius temperature-controlled environment and monitored for five days. The minnows that expired during this time were frozen in plastic bags. After the five days the remaining fish were terminated by exposure. The minnows were converted to ashes in a muffle furnace and prepared following the Yankton Biological Research Field Stations SOP F6.3.13. The samples were then analyzed by an atomic absorption spectrophotometer 200-A. The results showed a substantial difference between the control and experimental groups, thus rendering our hypothesis as accurate.
Keywords: Selenium absorption, Marine and freshwaters ecosystem, Fathead minnows’ ashes, Undergraduate research, and atomic absorption spectrophotometer
Cite this paper: Shahrokh Ghaffari, Shadi Abu-Baker, Erin Glynn, Accumulation of Selenium in Fathead Minnows (Pimrphales Promelas), American Journal of Environmental Engineering, Vol. 11 No. 1, 2021, pp. 13-15. doi: 10.5923/j.ajee.20211101.02.
Article Outline
1. Introduction
- The purpose of conducting this undergraduate research study on the accumulation of selenium in fathead minnows were to discover the amount of selenium absorbed and thus accumulated in the food chain. Selenium is found in both marine and freshwaters [1]. Selenium can exist in multiple oxidation states such as selenate, Se (VI); selenite, Se (IV); elemental selenium, Se (0); and selenide, Se (-II). It can also be found in multiple chemical forms within an oxidation state, such as organic and inorganic selenide [2]. Selenium is necessary in small amounts, but it can have adverse and be potentially fatal if consumed in large quantities. Waterborne selenium with concentrations of 2 mg/L or greater should be considered hazardous to the health and long-term survival of fish and wildlife populations due to the high potential for food- chain bioaccumulation, dietary toxicity, and reproductive effects [3]. It is important to know how much bioaccumulation will take place in a particular species before estimating the dietary toxicity and reproductive effects. According to Lemly, field studies show that benthic invertebrates and certain forage fishes, such as fathead minnows, can accumulate 20 to 370 mg/g selenium and still maintain stable, reproducing populations. To analyze the amount of selenium found in the samples there are several recommended methods. They include hydride generation atomic absorption spectrometry, neutron activation analysis, fluorometry, and gas chromatography [4].
2. Methods and Results
2.1. Methods
- An This study was conducted using two 20-gallon fish aquariums filled with dechlorinated water. One tank was used as a control and the other as the experimental. The experimental tank had 20 ppm of sodium selenite, 99%, (Na2SeO3) added. Ten fathead minnows (Pimephales promelas, Figure 1) were placed in each tank. We picked this species as it was available used in literature before [4]. The water temperature was regulated to 20 degrees Celsius. The minnows were fed a few flakes of fish food every day. They were alive for five days. At the end of this time period the remaining fish were terminated by exposure. The fathead minnows were vacu-washed, freezed and the percent moisture of the samples is presented in Table 1. All the minnows were then ashed following the “Yankton Biological Research Field Stations SOP F6.3.13 procedure”. After the digestion of the minnows was completed, they were analyzed by an atomic absorption spectrophotometer 200-A. To produce a Calibration Curve four standards solution of selenium were prepared consisting of 2.5, 5, 10, and 25 ppm. The calculations required for the addition of selenium to conduct this study are as follows:Amount of Se added to experimental tank:3.4 L X 40 mg/L = 136 mg Se. Then to calculate Se in grams and ppm, (0.136 g Se / 46.6%) / 100 = 0.292 g Se 20 ppm = 0.292 g Se /2 = 0.146 g Se.
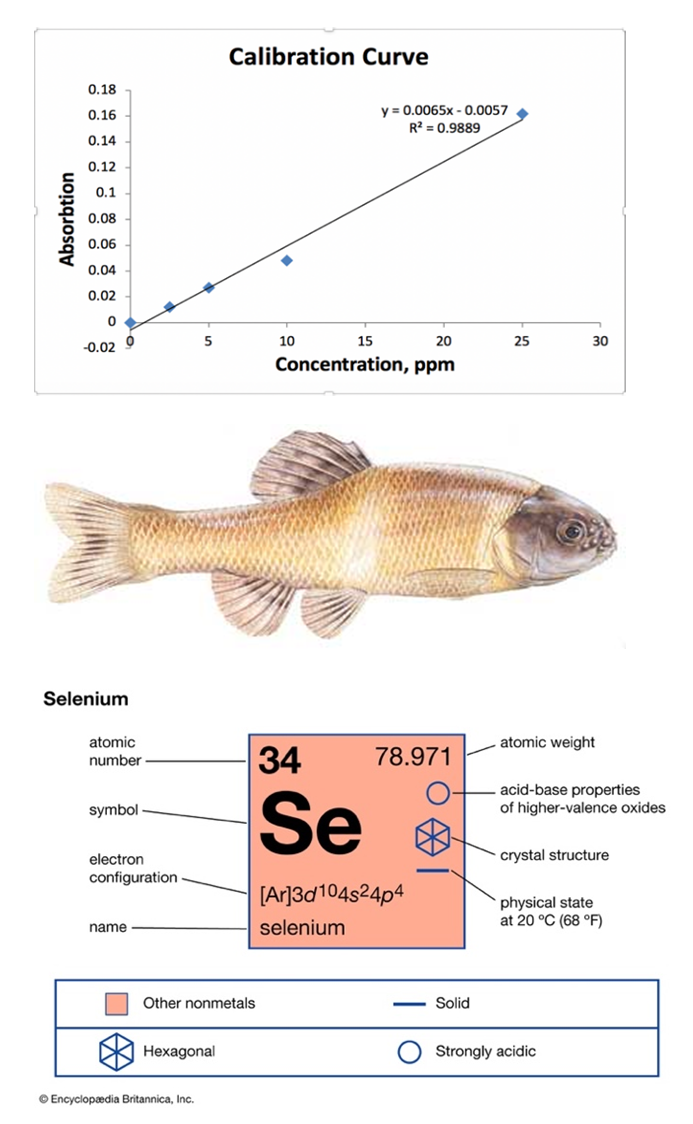 | Figure 1. Calibration curve of Se concentration in ppm vs. the absorbance, the image of Fathead Minnow (Pimephales promelas) and were obtained from online resource [6,7] |
2.2. Results
- The results indicate that the fathead minnows in the experimental tank did have a significant absorption of selenium in comparison with the control tank. The following table shows the concentrations and absorptions of the fish samples.
|
3. Discussion and Conclusions
- This experiment was a success in the fact that the hypothesis of increased selenium absorption in fathead minnows was accurate. According to Sanders, selenium is incorporated into the amino acids, and its transfer and depuration in the microbial food web may be analogous to those of nitrogen in some ways [1]. Thus, selenium is concentrated into the tissues of the fathead minnows. This value was measured by the atomic absorption spectrophotometer and indicates the increased amount of selenium found in the experimental tank compared to the control tank. "Most selenium accumulation occurs at the base of the food web and selenium concentration in predators generally reflects the concentration in their prey" [1]. The fathead minnows used in this study would be considered to be close to the base of the food web and therefore they would bioaccumulate selenium at a higher concentration than a larger predator. The chemistry of the water ecosystem also reflects the habitat of fish. The ecosystem used in this experiment was very narrow and selective. "Scientists now understand that water chemistry and biochemical transformations of selenium influence its accumulation in the tissues of aquatic life-forms-and therefore determine its impact on the ecology of lakes and streams" [2]. By understanding the cycling of various chemicals in the ecosystem it is easier to predict the impact an increased amount of a certain chemical might have. For example, the increased amount of selenium found in the experimental tank had an adverse effect on the minnows by causing death in a short amount of time. This was not a gradual increase, but a more dramatic increase and thus detrimental. This research was conducted to discover and evaluate what an increased amount of selenium in the water supply would accumulate in the body tissue of fathead minnows. It has been reported that acute toxicity has been seen in regions with high selenium content in food, and the main cellular toxopathology is not currently well understood. Some studies suggested mechanisms including the formation of superoxide and hydroxyl anions, together with hydrogen peroxide [8]. This study could expand to include toxicity studies of sodium selenite and sodium selenate acute toxicity other species as reported [9]. We could also study the effect of different concentrations of selenium on different species of fish as reported in the literature [10].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML